Introduction to Tartaric Acid
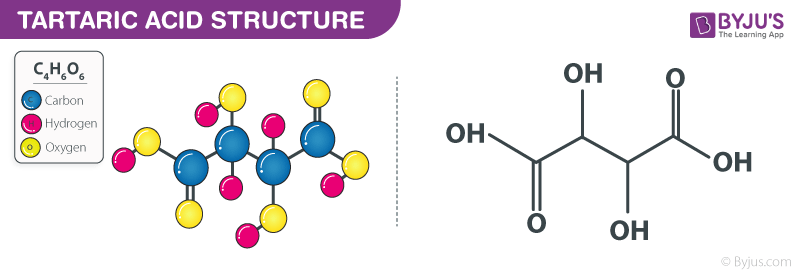
Tartaric acid is a naturally occurring organic acid that is commonly found in many fruits, particularly in grapes. It is a dicarboxylic acid, meaning it contains two carboxyl groups (COOH) in its chemical structure. Tartaric acid has the chemical formula C4H6O6 and a molecular weight of 150.09 g/mol.
Tartaric acid is widely used in various industries due to its unique properties and versatile applications. In the food and beverage industry, it is primarily utilized as a flavoring agent, acidulant, and pH regulator. It is responsible for the tart taste in fruits, such as grapes, and is often added to food products to enhance their flavor. Tartaric acid is commonly used in the production of wines, as it helps to control the acidity and stabilize the color and taste.
In addition to its uses in the food industry, tartaric acid also finds applications in the pharmaceutical and cosmetic industries. It is utilized as an excipient in medication formulations, acting as a chelating agent and helping to enhance the solubility of certain drugs. Tartaric acid is also found in various skincare and haircare products, as it helps to balance pH levels, improve skin texture, and promote exfoliation.
Tartaric acid is a white crystalline powder that is soluble in water and ethanol. It has a melting point of around 168-170 °C and a boiling point of 399 °C. It has a strong acidic taste and is typically available in the form of its salts, such as potassium bitartrate (cream of tartar).
Overall, tartaric acid is an important compound in the field of chemistry due to its wide range of applications and properties. Its unique chemical structure and acidity make it a valuable ingredient in various industries, from food and beverages to pharmaceuticals and cosmetics.
Chemical Composition of Tartaric Acid (C₄H₄O₆)
Tartaric acid has the chemical formula C₄H₄O₆. It is an organic acid that is naturally found in many fruits, particularly grapes. The chemical composition of tartaric acid consists of four carbon (C) atoms, four hydrogen (H) atoms, and six oxygen (O) atoms. It has a molecular weight of 150.09 grams per mole.
Properties and Uses of Tartaric Acid
Tartaric acid is a diprotic organic acid commonly found in many fruits, particularly grapes. It has various properties and uses in chemistry, including:
1. Acidic nature: Tartaric acid is a strong acid, meaning it readily donates protons or hydrogen ions in solution. This property allows it to participate in acid-base reactions and contribute to the overall acidity of a solution.
2. Chirality: Tartaric acid has two chiral centers, resulting in four possible stereoisomers. This property makes it valuable in asymmetric synthesis and as a resolving agent for separating enantiomers.
3. Buffering agent: Tartaric acid can act as a buffering agent, helping to maintain the pH of a solution within a specific range. Buffers containing tartaric acid are often used in food and beverage processing.
4. Food additive: Tartaric acid is commonly used as a food additive, primarily as an acidulant and flavor enhancer. It is found in many food products, including soft drinks, jams, jellies, and candies.
5. Wine production: Tartaric acid is a natural component of grapes and plays a crucial role in wine production. It contributes to the acidity, flavor, and stability of wine and is often added to adjust the pH and enhance tartness.
6. Cleaning agent: Tartaric acid is known for its ability to dissolve and remove calcium deposits or scales. It is often used as an ingredient in cleaning products such as descalers and dishwasher cleaners.
7. Medicinal applications: Tartaric acid is used in the pharmaceutical industry as an excipient, helping to improve the stability, solubility, and bioavailability of certain drugs. It is also employed as a chelating agent to enhance the absorption of certain minerals in the body.
8. Electroplating: Tartaric acid is sometimes added to electroplating baths to improve the quality and uniformity of metal coatings. It acts as a complexing agent, preventing the precipitation of metal salts and ensuring a smooth plating process.
9. Cosmetic formulations: Tartaric acid can be found in various cosmetic products, such as facial cleansers, masks, and peels. It is used to exfoliate and smooth the skin by breaking down and removing dead cells, revealing a fresher complexion.
Overall, tartaric acid has a wide range of applications in chemistry, food and beverage industries, pharmaceuticals, and various other fields.
Production and Sources of Tartaric Acid
Tartaric acid is a naturally occurring organic acid found in many fruits such as grapes, bananas, and tamarinds. It is also present in wine, as it is formed during the fermentation process. Tartaric acid is produced commercially through a combination of natural sources and chemical synthesis.
Natural sources of tartaric acid include grapes, which are the primary source due to their high tartaric acid content. After grape juice is fermented to produce wine, a sediment called wine lees is formed. This sediment contains a solid form of tartaric acid known as potassium bitartrate, or cream of tartar. This cream of tartar can be purified and converted into tartaric acid through chemical processes.
Another natural source of tartaric acid is tamarind, a tropical fruit. The acidic pulp of tamarind contains a significant amount of tartaric acid. Tamarind can be processed to extract tartaric acid for commercial use.
In addition to these natural sources, tartaric acid can also be synthesized chemically. The synthetic production involves the reaction between maleic anhydride and glycerol or through the oxidation of tartaric acid derivatives like tartaric acid ethyl ester.
Overall, tartaric acid is produced both from natural sources such as grapes and tamarind, as well as through chemical synthesis methods.
Health Effects and Safety Considerations of Tartaric Acid
Tartaric acid is a naturally occurring organic acid that is commonly used in the food and beverage industry as a flavoring agent, acidity regulator, and stabilizer. It is also found in many fruits, such as grapes, bananas, and citrus fruits. When consumed in moderate amounts, tartaric acid is generally considered safe for most individuals. However, it is important to be aware of its potential health effects and safety considerations.
Health Effects:
1. Gastrointestinal Discomfort: Consuming excessive amounts of tartaric acid may cause gastrointestinal discomfort, including stomach cramps, diarrhea, and abdominal pain. Individuals with sensitive digestive systems or pre-existing gastrointestinal conditions may be more susceptible to these effects.
2. Allergic Reactions: Some individuals may be allergic to tartaric acid, leading to symptoms such as itching, skin rashes, swelling, or difficulty breathing. If you experience any allergic reactions after consuming tartaric acid, it is advisable to seek medical assistance immediately.
3. Tooth Erosion: Tartaric acid is acidic in nature and may contribute to tooth erosion when consumed in large quantities or in highly concentrated forms. This can lead to dental issues such as tooth sensitivity or cavities. It is recommended to practice good oral hygiene and limit the consumption of tartaric acid-containing products to prevent tooth erosion.
Safety Considerations:
1. Dose Limitations: The Acceptable Daily Intake (ADI) for tartaric acid is set by regulatory authorities to ensure its safe consumption. For tartaric acid, the ADI is 30 mg/kg of body weight per day. It is important to moderate the intake of tartaric acid and not exceed the recommended daily dose to avoid potential health risks.
2. Interaction with Medications: Tartaric acid may interact with certain medications, such as antacids or medications used to treat urinary tract infections or kidney stones. It is advisable to consult a healthcare professional if you are taking any medications to ensure there are no potential interactions.
3. Safe Handling and Storage: Tartaric acid should be handled and stored in accordance with safety guidelines. It is important to wear appropriate protective equipment, such as gloves and safety goggles, when handling tartaric acid in its concentrated form. Additionally, it should be stored in a cool, dry place and kept out of reach of children and pets.
Overall, tartaric acid is generally considered safe for consumption in moderate amounts. However, individuals with specific health conditions or concerns should consult a healthcare professional before consuming products containing tartaric acid. It is important to read product labels and follow recommended dosage guidelines to ensure safe use.

Abigail Gutmann Doyle is a renowned Organic chemistry professor in Los Angeles. Her research focuses on the development of new chemical transformations in organic chemistry. She has won awards such as: Bayer Early Excellence in Science Award, Phi Lambda Upsilon National Fresenius Award, Presidential Early Career Award for Scientists and Engineers, BMS Unrestricted Grant in Synthetic Organic Chemistry.