Introduction to Planck’s radiation law
Planck’s radiation law, also known as Planck’s black body radiation law, is a fundamental principle in physics that describes the intensity and distribution of electromagnetic radiation emitted by a perfect black body at a given temperature.
In the late 19th century, scientists were studying the emission of radiation from objects as they were heated. They observed that the emitted radiation did not match the predictions of classical physics. Classical physics, based on the principles of thermodynamics and electromagnetism, predicted that the intensity of radiation emitted by an object would continually increase as its temperature increased, leading to the “ultraviolet catastrophe.”
Max Planck, a German physicist, proposed a groundbreaking theory in 1900 to explain this discrepancy. He suggested that electromagnetic radiation can only be emitted in discrete packets of energy, which he called “quanta” or “energy quanta.” This concept formed the foundation of quantum mechanics, a theory that revolutionized our understanding of the microscopic world.
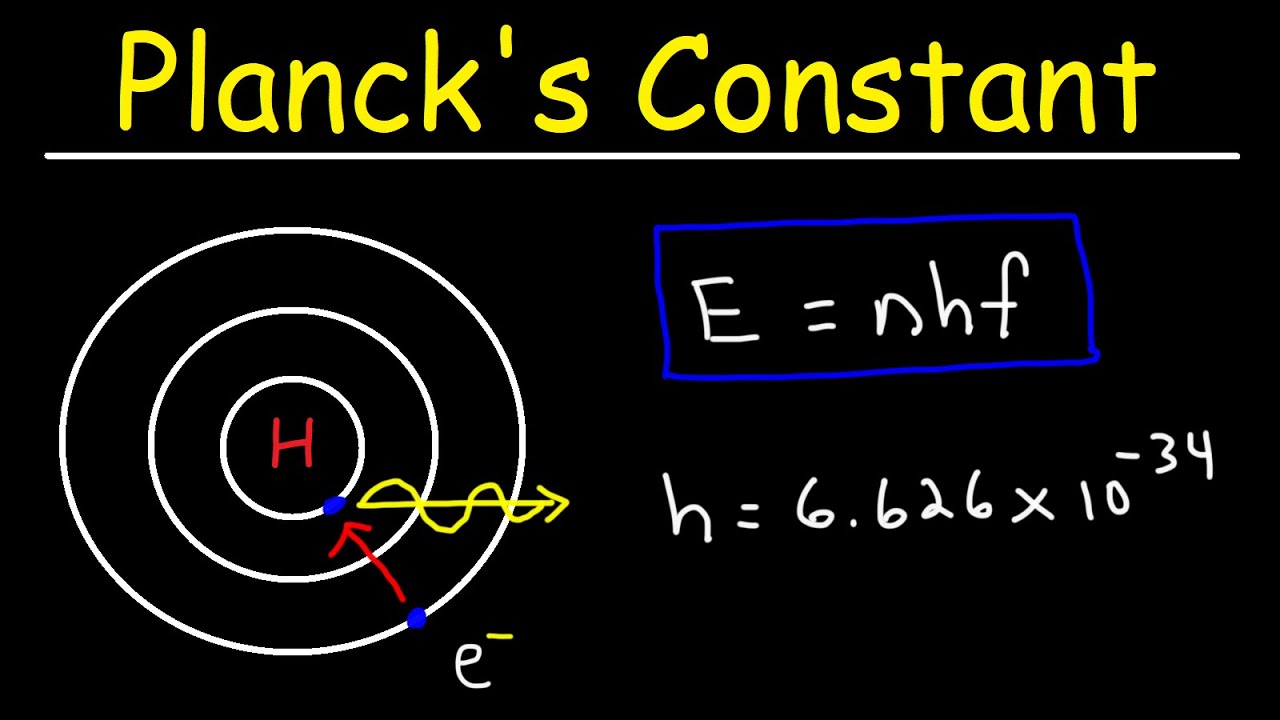
According to Planck’s radiation law, the energy E of each quantum of radiation is directly proportional to its frequency ν: E = hν. Here, h is the Planck constant, a fundamental constant in quantum mechanics.
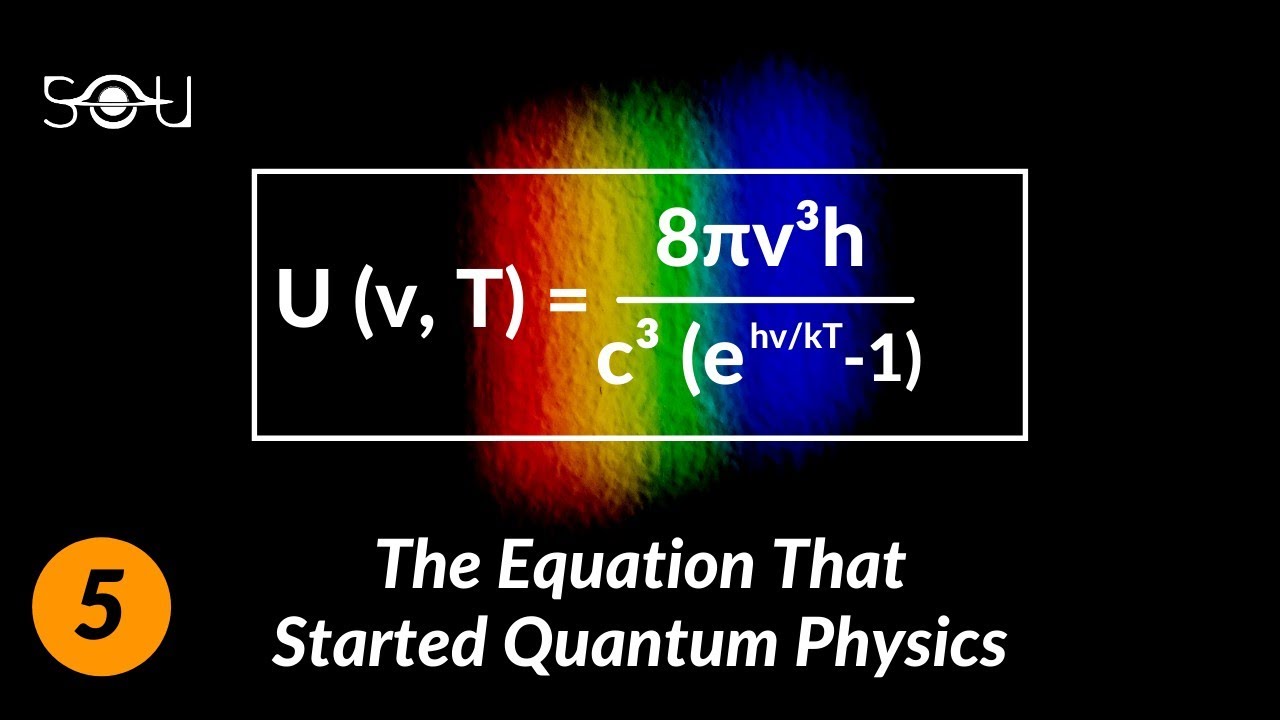
Planck further developed a formula to describe the spectral energy density, or the amount of energy radiated per unit volume and unit frequency range, by a perfect black body at a given temperature. The formula is known as Planck’s radiation law and is given by:
B(ν, T) = (2hν^3 / c^2) * (1 / (e^(hν / (kT)) – 1))
In this formula, B(ν, T) represents the spectral energy density, ν is the frequency of the radiation, T is the temperature of the black body, c is the speed of light, and k is the Boltzmann constant.
Planck’s radiation law successfully explained the observed distribution of radiation emitted by objects at different temperatures, including the classical predictions at low frequencies and the new quantum predictions at high frequencies. It laid the foundation for the development of quantum mechanics and earned Planck the Nobel Prize in Physics in 1918.
Overall, Planck’s radiation law is a fundamental principle that describes the emission of radiation from objects based on the discrete nature of energy associated with electromagnetic radiation.
Explanation of black body radiation
Black body radiation refers to the electromagnetic radiation emitted by an idealized object known as a black body. A black body is an object that perfectly absorbs all radiation that falls on it and also emits radiation at all wavelengths.
According to Planck’s radiation law, the intensity of radiation emitted by a black body at a specific wavelength and temperature is given by the formula:
I(λ, T) = (2hc²/λ⁵) * (1 / (e^(hc / λkT) – 1))
In this formula:
– I(λ, T) represents the spectral radiance (intensity per unit wavelength) of the radiation at a specific temperature T and wavelength λ.
– h is Planck’s constant (approximately 6.626 x 10^-34 J·s),
– c is the speed of light in a vacuum (approximately 2.998 x 10^8 m/s),
– λ is the wavelength of the radiation,
– k is the Boltzmann constant (approximately 1.381 x 10^-23 J/K), and
– T is the absolute temperature of the black body.
Planck’s radiation law reveals that black body radiation is not continuous, but rather exhibits a specific distribution of intensities at different wavelengths for each temperature. The curve representing this distribution is known as the Planck’s black body radiation curve.
Interestingly, Planck’s radiation law is fundamental in understanding various phenomena, such as the ultraviolet catastrophe, which classical physics failed to explain. It also played a crucial role in the development of quantum mechanics, as it was one of the first instances where quantization of energy was introduced.
Discovering Planck’s radiation law
Planck’s radiation law, also known as Planck’s law of black body radiation, is a fundamental principle in physics that describes the emission of electromagnetic radiation from a perfectly black body at a given temperature. Max Planck, a German physicist, formulated this law in 1900 to explain the observed distribution of energy emitted by such objects.
According to Planck’s radiation law, the intensity or power radiated by a black body at a specific wavelength, denoted by λ, is given by the formula:
I(λ, T) = (2hc²/λ⁵) × (1/(e^(hc/λkT) – 1))
Where:
– I(λ, T) is the radiation intensity at wavelength λ and temperature T.
– h is Planck’s constant, which relates the energy of a photon to its frequency (h = 6.62607015 × 10^-34 J·s).
– c is the speed of light in a vacuum (c ≈ 3 × 10^8 m/s).
– k is the Boltzmann constant, which relates temperature to energy (k ≈ 1.380649 × 10^-23 J/K).
– T is the temperature of the black body (in Kelvin).
Planck’s law plays a crucial role in quantum physics, as it introduced the concept of quantization of energy. Planck proposed that electromagnetic radiation is emitted in discrete packets of energy, called quanta or photons. This revolutionary idea laid the foundation for the development of quantum mechanics and the understanding of the particle-like behavior of light.
Through Planck’s radiation law, scientists were able to accurately explain the phenomenon of black body radiation, which had previously been a challenge for classical physics. The law accurately predicted the observed radiation spectra across a wide range of temperatures and wavelengths, making it a significant breakthrough in understanding the behavior of electromagnetic radiation.
Mathematical formulation of Planck’s radiation law
Planck’s radiation law is a mathematical expression that describes the intensity spectrum of blackbody radiation at a given temperature. The law can be formulated as follows:
I(λ, T) = (2hc²/λ⁵) * (1 / (exp(hc/λkT) – 1))
In this equation, I(λ, T) represents the intensity of radiation at a specific wavelength (λ) and temperature (T). The Planck constant is denoted by h, the speed of light by c, and the Boltzmann constant by k. The equation calculates the energy per unit time emitted at each wavelength by a blackbody radiator at a specific temperature.
This formulation of Planck’s radiation law accurately describes the distribution of energy across different wavelengths for a blackbody at any given temperature.
Applications and significance of Planck’s radiation law
Planck’s radiation law is a fundamental principle in physics that relates the intensity and wavelength distribution of electromagnetic radiation emitted by black bodies, which are objects that absorb all incident radiation. The law provides insights into the behavior of light and has several applications and significance in various scientific fields. Here are some examples:
1. Quantum mechanics: Planck’s radiation law played a crucial role in the development of quantum mechanics. In order to explain the observed intensity distribution of black body radiation, Planck introduced the concept of energy quantization, where energy is emitted or absorbed in discrete packets (quanta). This idea laid the foundation for understanding the discrete nature of energy levels in atoms and the quantum nature of light.
2. Astrophysics: Planck’s radiation law is utilized in astrophysics to study the thermal radiation emitted by celestial objects. By analyzing the intensity and spectral characteristics of radiation from stars and galaxies, scientists can determine their composition, temperature, and other fundamental properties. The law also helps in understanding the thermal equilibrium of bodies in space and the Big Bang theory.
3. Thermal radiation: Planck’s radiation law is important in studying thermal radiation emitted by objects at everyday temperatures. It provides a mathematical description of the distribution of energies and wavelengths of photons emitted by a hot object, allowing scientists to analyze and predict heat transfer, radiative cooling, and other thermal processes.
4. Energy-efficient lighting: The study of Planck’s radiation law has contributed to the development of energy-efficient lighting sources, such as light-emitting diodes (LEDs). By understanding the emission spectra and color temperatures associated with different materials, scientists can design LEDs that produce the desired lighting with minimal energy consumption.
5. Earth’s energy balance: Planck’s radiation law is utilized in climate science to study the Earth’s energy balance. It helps in modeling and understanding the distribution of thermal radiation emitted by the Earth’s surface, atmosphere, and clouds. This has implications for studying the greenhouse effect, climate change, and global warming.
In summary, Planck’s radiation law has widespread applications and significance in various fields of science, including quantum mechanics, astrophysics, thermal radiation, energy-efficient lighting, and climate science. It provides essential insights into the behavior of electromagnetic radiation, contributing to our understanding of the fundamental nature of light and its interactions with matter.
Topics related to Planckʼs Radiation Law
Derivation of Planck's Black Body Radiation #shorts #physics – YouTube
Derivation of Planck's Black Body Radiation #shorts #physics – YouTube
Quantization of Energy Part 1: Blackbody Radiation and the Ultraviolet Catastrophe – YouTube
Quantization of Energy Part 1: Blackbody Radiation and the Ultraviolet Catastrophe – YouTube
Planck's Constant and BlackBody Radiation – YouTube
Planck's Constant and BlackBody Radiation – YouTube
Understanding Black Body Radiation, Rayleigh-Jeans Law, & Ultraviolet Catastrophe – Quantum Physics – YouTube
Understanding Black Body Radiation, Rayleigh-Jeans Law, & Ultraviolet Catastrophe – Quantum Physics – YouTube
Blackbody Radiation and Wien's Law – YouTube
Blackbody Radiation and Wien's Law – YouTube
Deriving Planck's Law | The Equation That Began Quantum Physics – YouTube
Deriving Planck's Law | The Equation That Began Quantum Physics – YouTube
Planck Radiation Law – A quantum approach | In Hindi – YouTube
Planck Radiation Law – A quantum approach | In Hindi – YouTube
Planck's Law Derivation #physics #iit #gate – YouTube
Planck's Law Derivation #physics #iit #gate – YouTube
Quantum Physics – Part 1 (Blackbody radiation, Wien's Displacement Law, Planck's Law) – YouTube
Quantum Physics – Part 1 (Blackbody radiation, Wien's Displacement Law, Planck's Law) – YouTube
Quantum mechanics 04: Planck's Radiation law|Applied Physics – YouTube
Quantum mechanics 04: Planck's Radiation law|Applied Physics – YouTube

Konstantin Sergeevich Novoselov is a Russian-British physicist born on August 23, 1974. Novoselov is best known for his groundbreaking work in the field of condensed matter physics and, in particular, for his co-discovery of graphene. Novoselov awarded the Nobel Prize in Physics. Konstantin Novoselov has continued his research in physics and materials science, contributing to the exploration of graphene’s properties and potential applications.