Introduction
Introduction:
In the field of physics and thermodynamics, there are several fundamental laws that explain the behavior of gases under different conditions. Three such laws are Boyle’s Law, Mariotte’s Law, and Charles’s Law. These laws are essential for understanding the relationship between the pressure, volume, and temperature of a gas.
Boyle’s Law:
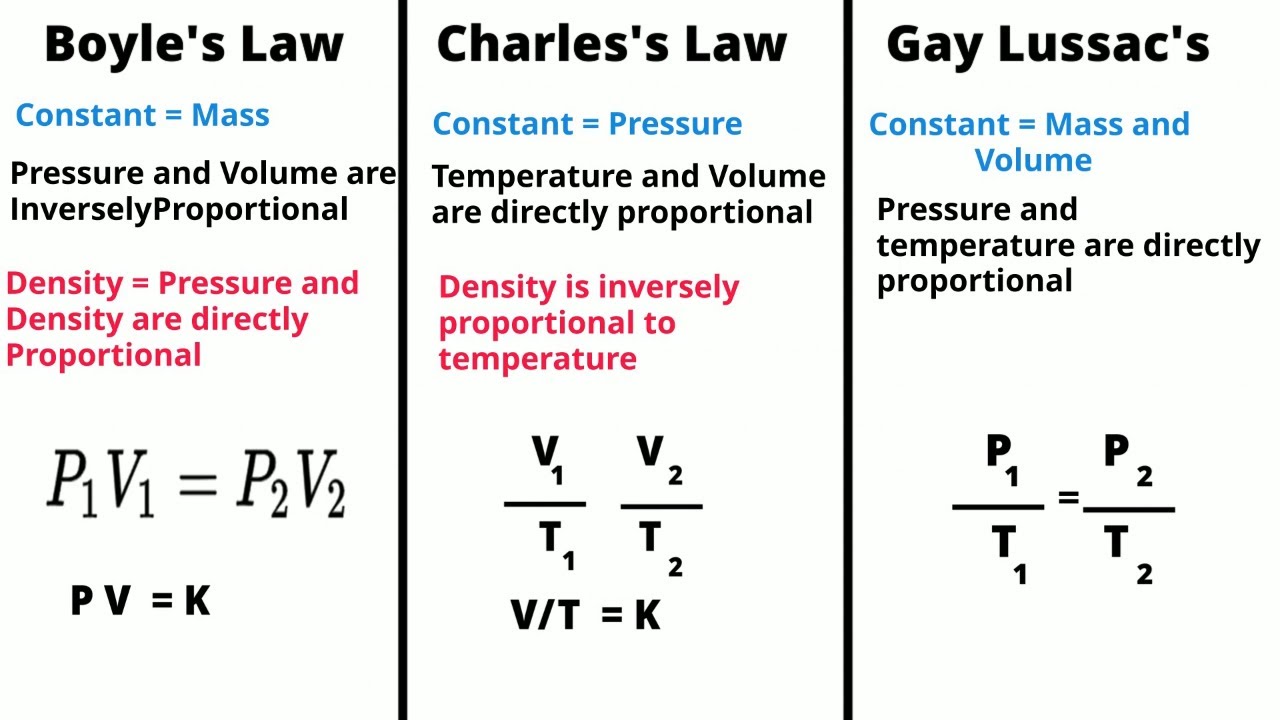
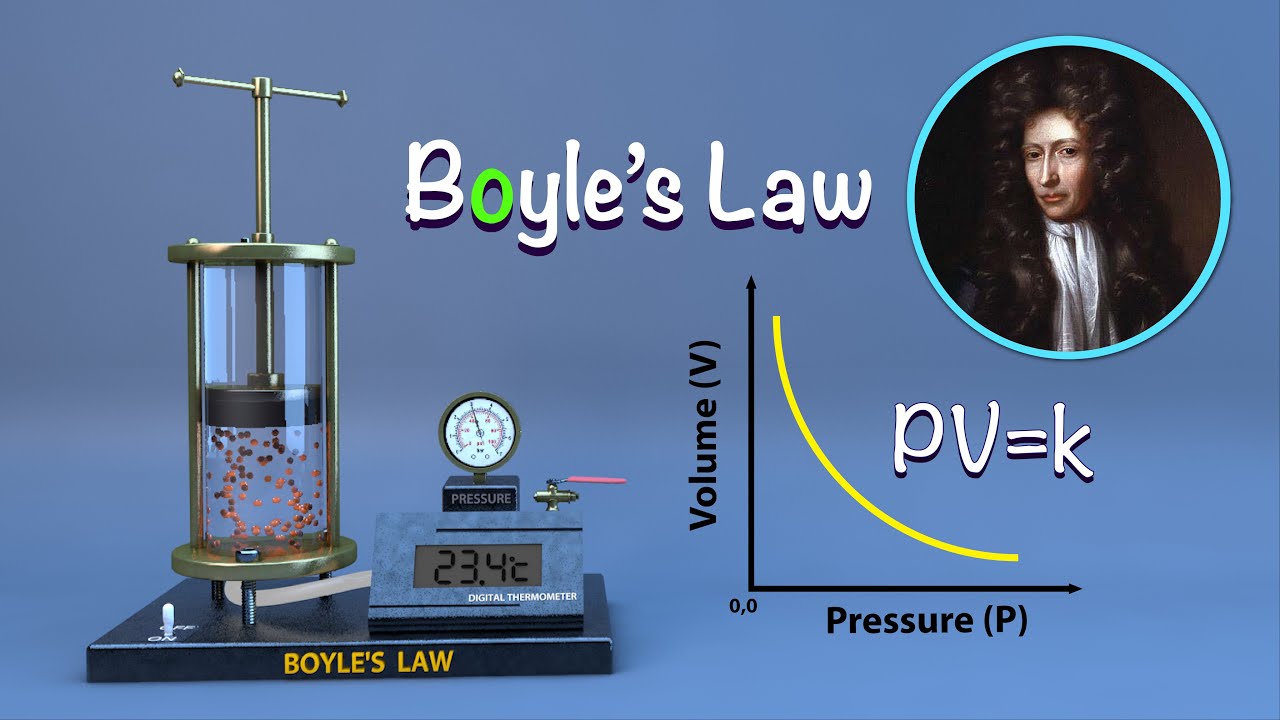
Boyle’s Law, named after the Irish physicist Robert Boyle, states that at a constant temperature, the pressure of a gas is inversely proportional to its volume. In other words, as the volume of a gas increases, its pressure decreases, and vice versa, as long as the temperature remains constant. Mathematically, this relationship can be expressed as P1V1 = P2V2, where P1 and V1 are the initial pressure and volume, and P2 and V2 are the final pressure and volume.
Mariotte’s Law:
Mariotte’s Law, also known as Boyle-Mariotte’s Law, is the French version of Boyle’s Law, named after the French physicist Edme Mariotte. This law is essentially the same as Boyle’s Law and describes the relationship between pressure and volume, but with some historical differences in how it was derived. Mariotte’s Law states that for a given amount of gas at a constant temperature, the product of the pressure and volume is constant. This can be expressed as P1V1 = P2V2, where P1 and V1 are the initial pressure and volume, and P2 and V2 are the final pressure and volume.
Charles’s Law:
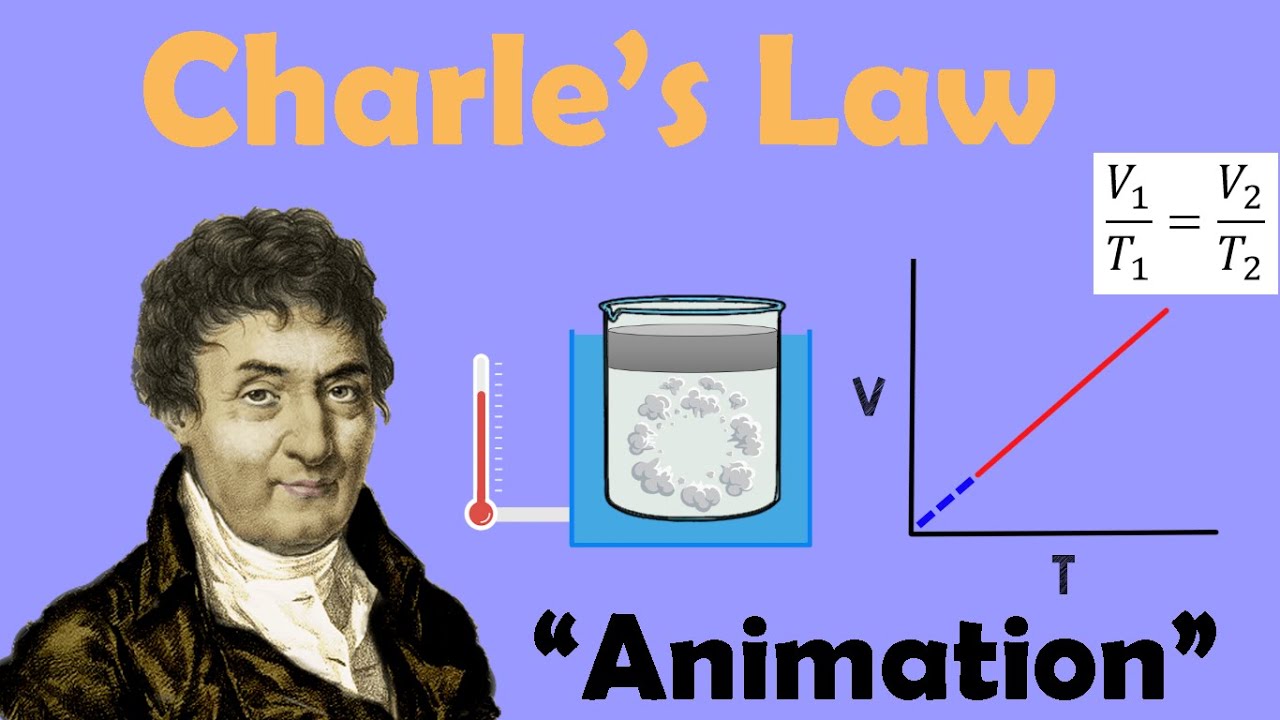
Charles’s Law, named after the French physicist Jacques Charles, states that the volume of a gas is directly proportional to its temperature, at a constant pressure. In simple terms, as the temperature of a gas increases, its volume also increases, and vice versa, as long as the pressure remains constant. Mathematically, this relationship can be expressed as V1/T1 = V2/T2, where V1 and T1 are the initial volume and temperature, and V2 and T2 are the final volume and temperature.
Overall, Boyle’s Law, Mariotte’s Law, and Charles’s Law provide fundamental principles to understand the behavior of gases. These laws help scientists and engineers in various fields to make accurate predictions and calculations related to the pressure, volume, and temperature changes of gases.
Boyle’s Law
Boyle’s Law and Boyle-Mariotte’s Law are two different but related concepts in the field of physics. They both describe the behavior of gases under different conditions, specifically in relation to changes in pressure and volume.
Boyle’s Law, named after the Irish physicist Robert Boyle, states that at a constant temperature, the pressure of a given amount of gas is inversely proportional to its volume. Mathematically, this can be expressed as P1V1 = P2V2, where P1 and V1 represent the initial pressure and volume, and P2 and V2 represent the final pressure and volume.
Boyle-Mariotte’s Law is an alternative name for Boyle’s Law that is commonly used in French-speaking countries. It refers to the same principle as Boyle’s Law but is named after both Robert Boyle and the French physicist Edme Mariotte, who independently discovered this gas law.
On the other hand, Charles’s Law, named after the French physicist Jacques Charles, states that at a constant pressure, the volume of a gas is directly proportional to its temperature. This can be expressed as V1/T1 = V2/T2, where V1 and T1 represent the initial volume and temperature, and V2 and T2 represent the final volume and temperature.
These laws are fundamental in understanding the behavior of gases and are often applied in various scientific and engineering fields, such as in the study of thermodynamics, gas laws, and ideal gas behavior.
Mariotte’s Law
Mariotte’s Law, also known as Boyle’s Law or the Boyle-Mariotte-Charleʼs Law, states that for a fixed amount of gas at a constant temperature, the pressure and volume of the gas are inversely proportional to each other. In other words, if the volume of a gas increases, the pressure it exerts decreases, and vice versa, as long as the temperature remains constant.
Mathematically, this can be expressed as:
P₁V₁ = P₂V₂
where P₁ and V₁ are the initial pressure and volume of the gas, and P₂ and V₂ are the final pressure and volume.
Boyle’s Law is based on the observation that at a constant temperature, the product of pressure and volume for a given amount of gas remains constant. This law provides a fundamental understanding of the behavior of gases and is widely used in various applications, such as in scuba diving, gas cylinders, and air compressors.
It should be noted that the Boyle-Mariotte-Charleʼs Law combines Boyle’s Law with another fundamental gas law known as Charles’s Law, which states that at a constant pressure, the volume of a gas is directly proportional to its absolute temperature.
Together, these laws provide a comprehensive understanding of the relationship between pressure, volume, and temperature for a gas, forming the basis of the ideal gas law, which incorporates both Boyle’s and Charles’s Laws.
Charle’s Law
Charles’s Law is a gas law that describes the relationship between the volume and temperature of a gas, while Boyle-Mariotte’s Law describes the relationship between the pressure and volume of a gas. Together, they form the combined gas law, which incorporates both temperature and pressure changes.
Charles’s Law states that, at constant pressure, the volume of a gas is directly proportional to its temperature. This means that as the temperature of a gas increases, the volume will also increase, and vice versa, as long as the pressure remains constant. The law is expressed mathematically as V1/T1 = V2/T2, where V1 and T1 are the initial volume and temperature, and V2 and T2 are the final volume and temperature.
Boyle-Mariotte’s Law, on the other hand, describes the relationship between the pressure and volume of a gas, assuming the temperature remains constant. According to this law, the pressure of a gas is inversely proportional to its volume. In other words, as the pressure of a gas increases, the volume will decrease, and as the pressure decreases, the volume will increase. The law is expressed mathematically as P1V1 = P2V2, where P1 and V1 are the initial pressure and volume, and P2 and V2 are the final pressure and volume.
When combined, Charles’s Law and Boyle-Mariotte’s Law form the combined gas law, which allows for calculations involving changes in pressure, volume, and temperature. This law is expressed mathematically as (P1V1)/T1 = (P2V2)/T2, where P1, V1, and T1 are the initial pressure, volume, and temperature, and P2, V2, and T2 are the final pressure, volume, and temperature, respectively.
Conclusion
In conclusion, the Boyle-Mariotte-Charle’s Law describes the relationship between the pressure, volume, and temperature of a gas. According to Boyle’s Law, at a constant temperature, the pressure of a gas is inversely proportional to its volume. Mariotte’s Law states that at a constant volume, the pressure of a gas is directly proportional to its temperature. Lastly, Charle’s Law states that at a constant pressure, the volume of a gas is directly proportional to its temperature. These laws are fundamental in understanding the behavior of gases and have important applications in fields such as chemistry and physics.
Topics related to Boyle-Mariotte-Charleʼs Law
BOYLE'S LAW | Animation – YouTube
BOYLE'S LAW | Animation – YouTube
Boyle's Law Practice Problems – YouTube
Boyle's Law Practice Problems – YouTube
Boyle's Law Demonstrations – YouTube
Boyle's Law Demonstrations – YouTube
Gas Laws-Boyle's-Charles's-Gay Lussac's – YouTube
Gas Laws-Boyle's-Charles's-Gay Lussac's – YouTube
The law of Boyle and Mariotte – experiment – YouTube
The law of Boyle and Mariotte – experiment – YouTube
Boyle's, Charles's and the Pressure Law – A-level Physics Required Practicals – YouTube
Boyle's, Charles's and the Pressure Law – A-level Physics Required Practicals – YouTube
Boyle's Law – Physics A-level Required Practical – YouTube
Boyle's Law – Physics A-level Required Practical – YouTube
Boyle's law: Explanation, Limitations and Applications – Explained Details (Animation) – YouTube
Boyle's law: Explanation, Limitations and Applications – Explained Details (Animation) – YouTube
CHARLES' LAW | Animation – YouTube
CHARLES' LAW | Animation – YouTube
Boyle's Law – YouTube
Boyle's Law – YouTube

Konstantin Sergeevich Novoselov is a Russian-British physicist born on August 23, 1974. Novoselov is best known for his groundbreaking work in the field of condensed matter physics and, in particular, for his co-discovery of graphene. Novoselov awarded the Nobel Prize in Physics. Konstantin Novoselov has continued his research in physics and materials science, contributing to the exploration of graphene’s properties and potential applications.