Definition of Dalton’s Law of Partial Pressures
Dalton’s Law of Partial Pressures states that the total pressure exerted by a mixture of non-reacting gases is equal to the sum of the partial pressures of each individual gas. In other words, the total pressure of a mixture of gases is equal to the sum of the pressures that each gas would exert if it were present alone in the same volume and at the same temperature.
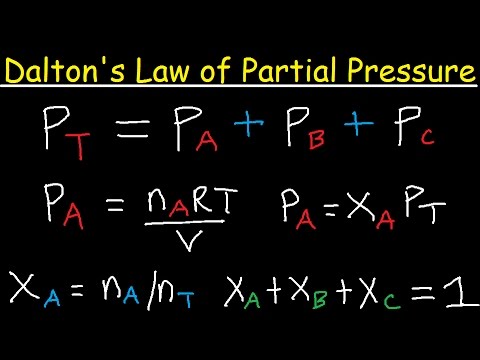
Mathematically, Dalton’s Law can be expressed as:
P total = P1 + P2 + P3 + … + Pn
where P total is the total pressure of the gas mixture, and P1, P2, P3, etc. are the partial pressures of the individual gases.
This law is based on the assumption that gases do not interact with each other and behave independently. It is commonly used in various fields such as chemistry, physics, and engineering to calculate the behavior and properties of gas mixtures.
Explanation of the concept of partial pressures
Partial pressures is a concept in chemistry that relates to the individual pressures exerted by different gases in a mixture.
Dalton’s Law of Partial Pressures states that the total pressure exerted by a mixture of gases is equal to the sum of the partial pressures of the individual gases. In other words, in a gas mixture, each gas behaves independently and does not interact with other gases.
To understand this concept, let’s consider a simple example. Suppose we have a mixture of two gases, A and B, in a container. The total pressure of the mixture is equal to the sum of the partial pressures of gases A and B.
Mathematically, Dalton’s Law can be expressed as:
Ptotal = PA + PB
Where Ptotal is the total pressure, PA is the partial pressure of gas A, and PB is the partial pressure of gas B.
The partial pressure of each gas is directly proportional to its mole fraction, which is the ratio of the number of moles of the gas to the total number of moles in the mixture. Therefore, we can also express Dalton’s Law as:
PA = XA * Ptotal
PB = XB * Ptotal
Where XA and XB are the mole fractions of gases A and B, respectively.
Dalton’s Law of Partial Pressures is particularly useful when dealing with mixtures of gases, as it allows us to determine the partial pressure of each gas based on its concentration and the total pressure of the mixture. Additionally, the law serves as a fundamental principle for many gas laws and gas-related calculations.
Mathematical formulation of Dalton’s Law
Dalton’s Law states that the total pressure exerted by a mixture of non-reacting gases is equal to the sum of the partial pressures of each individual gas. Mathematically, this can be expressed as:
P_total = P_1 + P_2 + P_3 + … + P_n
Where:
P_total is the total pressure exerted by the mixture
P_1, P_2, P_3, …, P_n are the partial pressures of each individual gas in the mixture.
Applications and examples of Dalton’s Law in physics
Dalton’s Law of Partial Pressures states that in a mixture of non-reacting gases, the total pressure exerted by the mixture is equal to the sum of the partial pressures exerted by each individual gas. This law has various applications and examples in physics and chemistry. Here are a few:
1. Gas mixtures in the atmosphere: The Earth’s atmosphere consists of a mixture of different gases, such as nitrogen, oxygen, carbon dioxide, and others. Dalton’s Law allows us to determine the partial pressure of each gas based on its concentration and total pressure. This is important in understanding factors like air pollution and gas exchange in biological systems.
2. Gas collection over water: When a gas is collected by displacing water in a laboratory setup, the presence of water vapor affects the total pressure. Dalton’s Law allows us to consider the partial pressure of the water vapor and subtract it from the total to determine the partial pressure of the collected gas.
3. Gas behavior in closed containers: When a container is filled with a mixture of gases, each gas exerts its own partial pressure. Dalton’s Law helps us understand how the total pressure inside the container is a result of the combined individual pressures of all the gases present.
4. Gas diffusion: Dalton’s Law can be used to explain the phenomenon of gas diffusion. According to this law, each gas in a mixture will diffuse from an area of higher partial pressure to an area of lower partial pressure. This concept is the basis of various gas separation techniques.
5. Gas behavior in chemical reactions: Dalton’s Law is relevant when gases are involved in chemical reactions. The partial pressures of reactant gases can determine the overall rate and outcome of a reaction. Additionally, Dalton’s Law is applied in gas stoichiometry to calculate the volumes of gases involved in chemical equations.
These are just a few examples of how Dalton’s Law is applied in physics and chemistry. The law provides a fundamental understanding of the behavior of gases in various scenarios and is widely used in gas-related calculations and experiments.
Limitations and assumptions of Dalton’s Law
Limitations of Dalton’s Law:
1. Dalton’s Law is only applicable to ideal gases: Dalton’s Law assumes that the gases are ideal, meaning they have negligible volume and do not interact with each other. In reality, gases deviate from ideality under certain conditions, such as at high pressures or low temperatures. This limitation makes the law less accurate for describing the behavior of real gases.
2. Dalton’s Law assumes that the total pressure exerted by a mixture of gases is equal to the sum of the partial pressures of the individual gases: However, this assumption is only valid as long as there are no intermolecular interactions or chemical reactions between the gases. If there are interactions or reactions occurring, the behavior of the gases may deviate from Dalton’s Law.
3. Dalton’s Law does not take into consideration the non-ideal behavior of real gases: Real gases can exhibit non-ideal behavior, such as molecular attractions and volume exclusion effects. Dalton’s Law does not account for these factors, which can lead to inaccuracies in predicting the behavior of gas mixtures.
Assumptions of Dalton’s Law:
1. The gases in the mixture behave independently: Dalton’s Law assumes that the gases in the mixture do not interact with each other and behave as if they are alone in the system. This assumption simplifies the calculations by treating each gas as if it occupies the entire volume of the container independently.
2. The total pressure is equal to the sum of the partial pressures: Dalton’s Law assumes that the total pressure exerted by a mixture of gases is simply the sum of the individual pressures exerted by each gas. This assumption is based on the idea that the gases do not interact with each other and each gas exerts its own pressure independently.
3. The gases are assumed to be ideal: Dalton’s Law assumes that the gases in the mixture are ideal, meaning they have no volume and do not interact with each other. This assumption allows for simpler calculations and predictions, but it is not accurate for real gases under all conditions.
Topics related to Daltonʼs Law of Partial Pressures
Daltons Law | Partial Pressures – YouTube
Daltons Law | Partial Pressures – YouTube
Dalton's Law of Partial Pressure Problems & Examples – Chemistry – YouTube
Dalton's Law of Partial Pressure Problems & Examples – Chemistry – YouTube
Dalton's Law of Partial Pressure Problems, Mole Fraction, Chemistry Gas Laws – YouTube
Dalton's Law of Partial Pressure Problems, Mole Fraction, Chemistry Gas Laws – YouTube
Dalton's Law of Partial Pressures Explained – YouTube
Dalton's Law of Partial Pressures Explained – YouTube
Partial Pressure | Dalton's Law of Partial Pressure – YouTube
Partial Pressure | Dalton's Law of Partial Pressure – YouTube
Dalton's Law of Partial Pressures Demonstration – YouTube
Dalton's Law of Partial Pressures Demonstration – YouTube
Real Gas and Ideal Gas – YouTube
Real Gas and Ideal Gas – YouTube
Boyle's Law | Chemistry – YouTube
Boyle's Law | Chemistry – YouTube
Ideal Gas Law Practice Problems – YouTube
Ideal Gas Law Practice Problems – YouTube
Gas Pressure: The Basics – YouTube
Gas Pressure: The Basics – YouTube

Konstantin Sergeevich Novoselov is a Russian-British physicist born on August 23, 1974. Novoselov is best known for his groundbreaking work in the field of condensed matter physics and, in particular, for his co-discovery of graphene. Novoselov awarded the Nobel Prize in Physics. Konstantin Novoselov has continued his research in physics and materials science, contributing to the exploration of graphene’s properties and potential applications.