Introduction to X-ray crystallography
X-ray crystallography is a powerful technique used in the field of structural biology to determine the three-dimensional arrangement of atoms within a crystal. It has revolutionized our understanding of the molecular world, providing detailed insights into the structure and function of a wide range of substances, including proteins, small organic molecules, and inorganic materials.
The principle behind X-ray crystallography relies on the ability of X-rays, a form of electromagnetic radiation, to interact with the electrons in atoms. When X-rays pass through a crystal, they scatter off the electrons in the crystal lattice, forming a diffraction pattern. By analyzing this diffraction pattern, scientists can deduce the arrangement of atoms within the crystal.
To obtain a diffraction pattern, a sample of the crystal is first prepared. The crystal must be of high quality, with well-defined and regularly arranged atoms. It is usually grown under controlled conditions using methods such as vapor diffusion or liquid-phase crystallization. Once a suitable crystal is obtained, it is mounted on a goniometer, which allows for precise rotational and translational movements.
The crystal is then exposed to a beam of X-rays generated by a specialized X-ray source, such as a synchrotron or an X-ray tube. The X-rays diffract off the crystal lattice, producing a pattern of spots on a detector located on the opposite side of the crystal. This diffraction pattern carries information about the intensities and angles of the scattered X-rays.
From the diffraction pattern, scientists employ mathematical algorithms and computational techniques to determine the electron density distribution within the crystal. By interpreting this electron density, they can derive the positions of the atoms in the crystal, as well as their chemical bonds and overall structure.
X-ray crystallography has played a crucial role in numerous scientific discoveries, such as the determination of the double helix structure of DNA by Rosalind Franklin and Maurice Wilkins. It has also facilitated the development of new drugs by revealing the three-dimensional shape of proteins and their interactions with potential drug compounds.
In recent years, X-ray crystallography has been enhanced by advances in technology and computational methods, allowing for the study of ever more complex and challenging structures. Additionally, the development of free-electron lasers has opened up new possibilities for studying structures that are difficult to crystallize or are highly dynamic.
Overall, X-ray crystallography remains a fundamental tool in structural biology, providing a detailed understanding of the atomic world and paving the way for the design of new materials and therapeutics.
Principles of X-ray crystallography
X-ray crystallography is a powerful technique used to determine the atomic and molecular structures of crystalline substances. It relies on the interaction of X-rays with the crystal lattice to produce a diffraction pattern, which can then be used to infer the arrangement of atoms within the crystal.
The principles of X-ray crystallography can be outlined as follows:
1. X-ray Generation: X-rays are produced using an X-ray generator, which typically involves accelerating electrons towards a target material. When the electrons collide with the target, X-rays are emitted.
2. Crystal Preparation: A pure crystal of the substance under study is required for X-ray crystallography. The crystal should be of good quality, with a regular and well-defined lattice structure. It is usually necessary to grow large, well-formed crystals for accurate analysis.
3. X-ray Diffraction: The crystal is placed in the path of the X-rays, and the X-rays interact with the atoms in the crystal lattice. This interaction leads to constructive and destructive interference of the X-rays, resulting in a pattern of dark and light spots known as a diffraction pattern. The diffraction pattern is recorded on a detector.
4. Data Collection: The diffraction pattern is captured by a detector, such as a photographic film or an electronic detector. The intensity and position of each spot of the diffraction pattern are recorded.
5. Data Analysis: The collected diffraction data is analyzed using mathematical techniques, such as Fourier transforms, to determine the spatial arrangement of the atoms within the crystal. The intensities and positions of the diffraction spots are used to calculate electron density maps, which represent the distribution of electrons in the crystal.
6. Model Building and Refinement: A model of the crystal structure is constructed based on the electron density map and the known chemical composition of the substance. The model is refined iteratively by adjusting the atomic positions and thermal vibrations until the calculated diffraction pattern matches the experimental data.
7. Structure Determination: Once the model is refined, the final crystal structure can be determined. This includes determining the positions of all atoms within the unit cell of the crystal, as well as their connectivity and chemical environments.
X-ray crystallography has revolutionized our understanding of molecular and material structures, providing valuable information in fields such as chemistry, biology, physics, and materials science. It allows scientists to visualize the complex three-dimensional arrangements of atoms and provides insight into the properties and behavior of crystalline substances.
Techniques and procedures in X-ray crystallography
X-ray crystallography is a widely used technique in determining the atomic and molecular structures of crystals. It relies on the diffraction of X-rays by the crystal lattice to obtain information about the arrangement of atoms within the crystal. Here are some of the key techniques and procedures involved in X-ray crystallography:
1. Crystallization: The first step in X-ray crystallography is to grow a well-ordered crystal of the substance being studied. This typically involves optimizing the conditions of temperature, solvent, pH, and concentration to promote crystal growth.
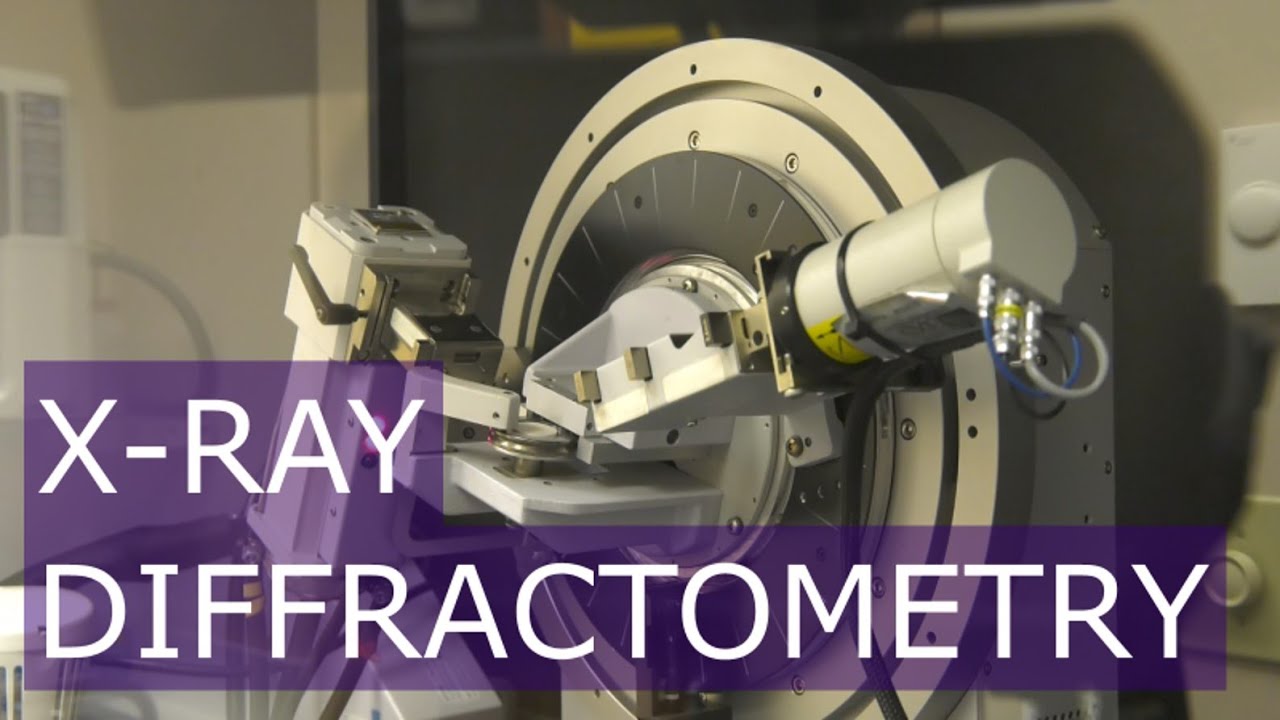
2. Data collection: Once a suitable crystal is obtained, X-ray diffraction data is collected by shining a beam of X-rays onto the crystal and measuring the scattered X-ray pattern. This is typically done using an X-ray diffractometer, which positions the crystal at different orientations to collect multiple diffraction images.
3. Data analysis: The collected diffraction data is then processed to determine the intensity and position of each diffracted spot. This involves correcting for instrumental effects, such as detector geometry and polarization, as well as background noise.
4. Indexing: The next step is to determine the indexing of the diffracted spots, which refers to assigning Miller indices to each spot. This determines the direction and length of the reciprocal lattice vectors and helps determine the unit cell dimensions and crystal symmetry.
5. Structure solution: The structure solution involves determining the phase angles of the diffracted spots, which are required to calculate the electron density of the crystal. Several methods, such as direct methods, molecular replacement, and isomorphous replacement, can be used to solve the phase problem.
6. Refinement: Once the initial electron density map is obtained, the structure is refined by adjusting the atomic positions and thermal vibrations to minimize the difference between the observed and calculated diffraction pattern. This process is typically performed using least-squares refinement methods.
7. Validation: After refinement, the structure is subjected to various validation checks to ensure its quality. This includes checking for correct atom positions, reasonable bond lengths, angles, and thermal parameters, and assessing the fit to the experimental data.
8. Reporting: The final step is to prepare a publication-quality report describing the crystal structure, including the atomic coordinates, bond lengths, angles, and other relevant parameters. This report is typically submitted to a scientific journal for publication.
X-ray crystallography has revolutionized our understanding of chemical structures and played a crucial role in fields such as materials science, drug discovery, and protein structure determination. It provides detailed atomic-level information, allowing scientists to design and optimize new materials and develop targeted drugs.
Applications of X-ray crystallography in physics
X-ray crystallography is a powerful technique used to analyze the atomic structure of a crystal. While it is commonly associated with the field of chemistry, it also finds several applications in the field of physics. Here are some of the key applications of X-ray crystallography in physics:
1. Materials Science: X-ray crystallography plays a crucial role in the study of materials at the atomic level. It helps determine the arrangement of atoms and their bonding within a crystal lattice, providing insights into the structure-property relationships of materials. This information is vital for understanding the behavior of materials and designing new materials with targeted properties.
2. Condensed Matter Physics: X-ray crystallography enables the investigation of the electronic and magnetic properties of crystalline materials. By analyzing the diffraction patterns produced by X-rays interacting with the crystal lattice, researchers can determine various characteristics such as the charge density, Fermi surfaces, and presence of magnetic ordering.
3. High-Pressure Physics: X-ray crystallography is used to study the behavior of materials under extreme pressure conditions. By subjecting crystals to high pressures and analyzing their diffraction patterns, scientists can gain insights into the structural changes and phase transitions that occur at such pressures. This knowledge helps in understanding the properties of materials in planetary interiors, for example.
4. Soft Matter Physics: X-ray crystallography can be applied to the study of soft materials, such as liquid crystals, polymers, and biological macromolecules. In this case, the technique is used to determine the arrangement and conformation of molecules within these materials. It helps elucidate the structure-function relationships and provides valuable information for the development of new materials or drugs.
5. Fundamental Physics: X-ray crystallography has played a significant role in fundamental physics research. For example, it has been used to determine the structure of viruses, proteins, and other biological macromolecules, aiding in the understanding of their function and the development of new drugs. It has also contributed to the discovery and characterization of various types of crystals, such as those displaying quasicrystalline or topological properties.
Overall, X-ray crystallography is an invaluable tool in physics, enabling the study of atomic and molecular structures, understanding material properties, and advancing scientific knowledge across various disciplines.
Challenges and future prospects of X-ray crystallography
X-ray crystallography is a widely used technique in the field of structural biology and materials science. It involves shooting X-rays at a crystal and analyzing the resulting diffraction pattern to determine the arrangement of atoms in the crystal. While X-ray crystallography has provided invaluable insights into the structure and function of a wide range of molecules, it also faces several challenges and has future prospects to overcome them.
One of the major challenges of X-ray crystallography is obtaining high-quality crystals suitable for analysis. Crystallization can often be a complex and time-consuming process, requiring optimization of various parameters such as pH, temperature, and precipitant concentration. Some molecules are inherently difficult to crystallize, and the quality of crystals obtained can vary greatly. This can limit the applicability of X-ray crystallography to certain systems.
Another challenge lies in the determination of the phases of the diffracted X-rays. This phase problem refers to the fact that the intensity of the X-rays can be measured, but the phase information is lost. Solving the phase problem is crucial for reconstructing the electron density map of the crystal. Various methods have been developed to tackle this problem, such as molecular replacement and experimental phasing techniques, but it remains a significant challenge, particularly for larger and more complex structures.
Additionally, X-ray crystallography is limited in its ability to provide real-time information about dynamic processes. As it requires the formation of stable crystals, the technique is generally limited to static structures. This restricts its use in studying molecular dynamics and conformational changes happening on short timescales. Alternative techniques, such as cryo-electron microscopy, are being developed to overcome this limitation.
The future prospects of X-ray crystallography involve addressing these challenges and improving the technique further. Advances in crystallization methods, such as the use of automated robotics and nanocrystallization techniques, are being explored to increase the success rate and quality of crystals obtained. Additionally, the development of advanced X-ray sources, such as X-ray free-electron lasers, has opened new possibilities for overcoming the phase problem and providing high-resolution data on nanosecond timescales.
Furthermore, combining X-ray crystallography with other structural biology techniques, such as nuclear magnetic resonance (NMR) spectroscopy and cryo-electron microscopy, can provide complementary information and enhance our understanding of complex biological systems. Integrative approaches, combining data from multiple techniques, are becoming increasingly important in pushing the boundaries of structural biology.
In conclusion, while X-ray crystallography has been a powerful tool for elucidating molecular structures, it still faces challenges such as crystallization difficulties and the phase problem. However, ongoing advancements and synergistic approaches have the potential to overcome these limitations and enhance the future prospects of X-ray crystallography in various fields of research.
Topics related to X-ray crystallography
What is X-ray Diffraction? – YouTube
What is X-ray Diffraction? – YouTube
What is Single Crystal X-ray Diffraction? – YouTube
What is Single Crystal X-ray Diffraction? – YouTube
crystallography and reciprocal space – YouTube
crystallography and reciprocal space – YouTube
21. X-ray Diffraction Techniques I (Intro to Solid-State Chemistry) – YouTube
21. X-ray Diffraction Techniques I (Intro to Solid-State Chemistry) – YouTube
Bragg's Equation For X-Ray Diffraction In Chemistry – Practice Problems – YouTube
Bragg's Equation For X-Ray Diffraction In Chemistry – Practice Problems – YouTube
Physics 307 Lab 7: Introduction to X-ray Physics and Diffraction – YouTube
Physics 307 Lab 7: Introduction to X-ray Physics and Diffraction – YouTube
X-Ray_Diffraction_of_DNA.f4v – YouTube
X-Ray_Diffraction_of_DNA.f4v – YouTube
X-Ray Diffraction (XRD) Basic Operation – YouTube
X-Ray Diffraction (XRD) Basic Operation – YouTube
X-Ray Diffraction by Bragg's Law – YouTube
X-Ray Diffraction by Bragg's Law – YouTube
Introduction to X-ray Diffraction – YouTube
Introduction to X-ray Diffraction – YouTube

Konstantin Sergeevich Novoselov is a Russian-British physicist born on August 23, 1974. Novoselov is best known for his groundbreaking work in the field of condensed matter physics and, in particular, for his co-discovery of graphene. Novoselov awarded the Nobel Prize in Physics. Konstantin Novoselov has continued his research in physics and materials science, contributing to the exploration of graphene’s properties and potential applications.