Introduction
Introduction:
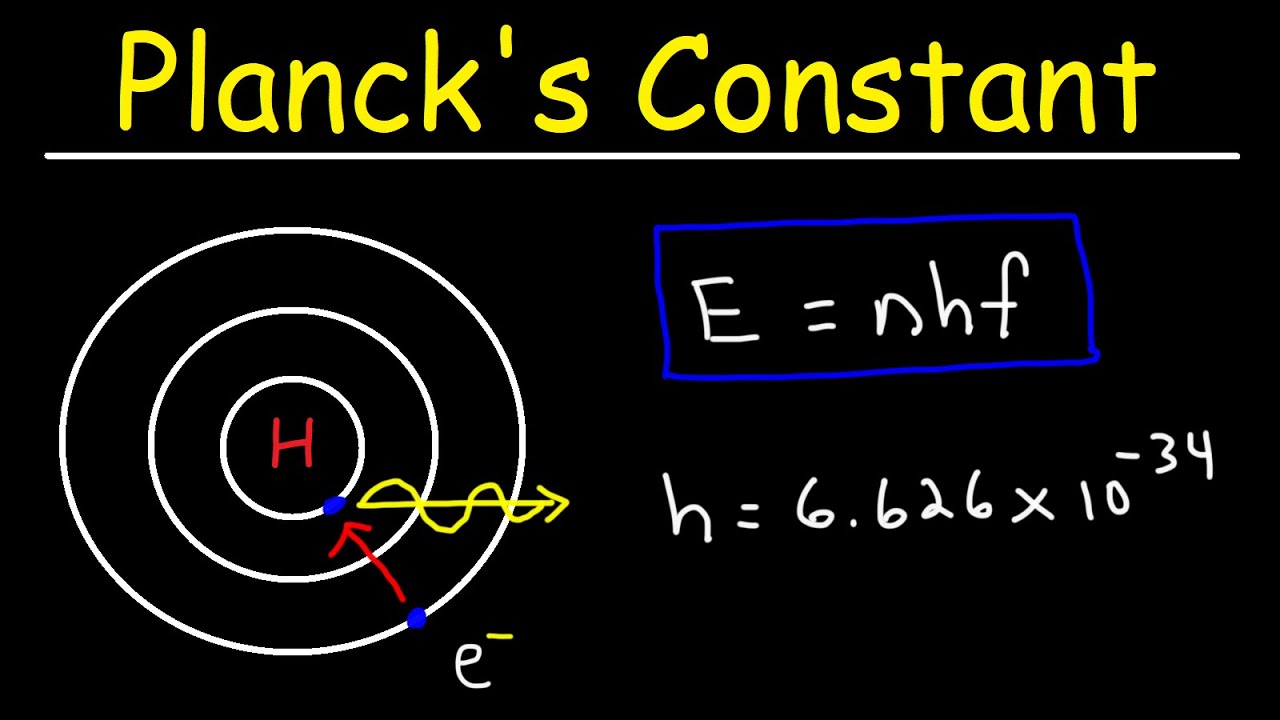
Planck’s radiation formula is an important contribution to the field of quantum mechanics. It was developed by physicist Max Planck in 1900 and provides a mathematical expression for the energy distribution of electromagnetic radiation at different wavelengths. This formula played a key role in the development of quantum theory, as it challenged the classical understanding of the behavior of light and paved the way for the concept of quantization.
Planck’s Radiation Formula:
Planck’s radiation formula can be expressed as:
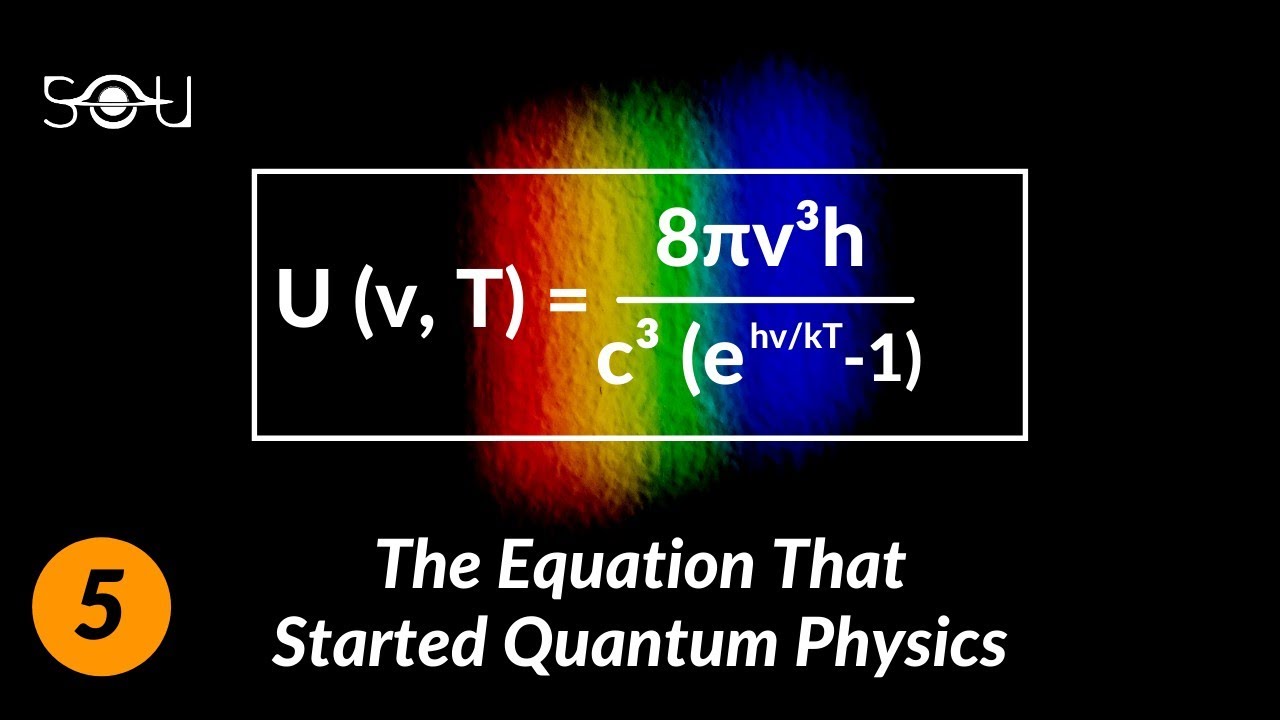
B(λ, T) = (2hc²/λ⁵) × [1/(e^(hc/λkT) – 1)]
In this formula, B(λ, T) represents the spectral radiance of a body at a given wavelength (λ) and temperature (T). The other constants used are h (Planck’s constant), c (the speed of light), and k (the Boltzmann constant).
Planck derived this formula by assuming that energy is quantized and can only be emitted or absorbed in discrete units called “quanta.” This idea revolutionized our understanding of the behavior of light, as it suggested that electromagnetic radiation is also quantized into discrete packets of energy, known as photons.
Planck’s radiation formula provides an accurate description of the energy distribution of black-body radiation, which is the electromagnetic radiation emitted by a perfectly absorbing object at thermal equilibrium. When applied to different temperatures, the formula allows us to predict the intensity and distribution of radiation emitted across the electromagnetic spectrum.
The success of Planck’s radiation formula in explaining the experimentally observed distributions of black-body radiation had a profound impact on the development of quantum mechanics. It challenged the classical wave theory of light and paved the way for the development of quantum theory by introducing the concept of quantization. Planck’s work laid the foundation for later breakthroughs in quantum mechanics, such as Albert Einstein’s explanation of the photoelectric effect and the development of the wave-particle duality concept.
Planck’s Radiation Formula explained
Planck’s Radiation Formula, also known as Planck’s blackbody radiation formula, is a mathematical equation that describes the spectral density of electromagnetic radiation emitted by a blackbody at a given temperature.
Max Planck derived this formula in 1900 while trying to understand the phenomenon of blackbody radiation. A blackbody is an object that absorbs all incident electromagnetic radiation and emits radiation across all wavelengths. Planck’s Radiation Formula calculates the amount of energy emitted per unit of time and per unit of wavelength (or frequency) by a blackbody.
The formula is given by:
[B(lambda, T) = frac{{2hc^2}}{{lambda^5}} cdot frac{1}{{e^{frac{{hc}}{{lambda k_B T}}} – 1}}]where:
– (B(lambda, T)) is the spectral radiance (power per unit area per unit solid angle per unit wavelength) at a specific wavelength (lambda) and temperature (T).
– (h) is the Planck constant (approximately (6.626 × 10^{-34} , text{J} cdot text{s})).
– (c) is the speed of light (approximately (3 × 10^8 , text{m/s})).
– (k_B) is the Boltzmann constant (approximately (1.38 × 10^{-23}, text{J/K})).
The formula includes the exponential term (frac{1}{{e^{frac{{hc}}{{lambda k_B T}}} – 1}}), which characterizes the distribution of energy in the emitted radiation. It accounts for the quantization of energy and describes the probability distribution of photons at different energy levels.
Planck’s Radiation Formula provides an accurate description of the radiation emitted by a blackbody across the entire electromagnetic spectrum. It successfully explained the observed behaviors of blackbody radiation and played a crucial role in the development of quantum mechanics by introducing the concept of energy quantization.
Significance of Planck’s Radiation Formula
Planck’s radiation formula is a fundamental equation in physics that describes the energy distribution of electromagnetic radiation emitted by a black body. It was formulated by the German physicist Max Planck in 1900 and is considered one of the most important contributions to the development of quantum theory.
The significance of Planck’s radiation formula lies in its ability to accurately describe the observed behavior of black bodies, which are objects that absorb and emit all radiation that falls on them. Prior to Planck’s work, scientists tried to explain black body radiation using classical physics, but these attempts were unsuccessful.
Planck introduced the concept of energy quantization, suggesting that the energy of electromagnetic radiation could only be emitted or absorbed in discrete packets, or “quanta”. This revolutionary idea laid the foundation for quantum mechanics and challenged the classical wave theory of light.
Planck’s formula mathematically represents how the intensity of radiation emitted by a black body depends on the wavelength of the radiation and the temperature of the black body. It accurately predicts the observed distribution of energies and wavelengths for different temperatures, matching experimental data across the electromagnetic spectrum.
Planck’s radiation formula also played a pivotal role in Albert Einstein’s explanation of the photoelectric effect, for which he later received the Nobel Prize. Einstein used Planck’s concepts of energy quantization to explain how light can exhibit both wave-like and particle-like properties.
Overall, Planck’s radiation formula was a groundbreaking development that revolutionized our understanding of the behavior of electromagnetic radiation and paved the way for the development of quantum physics. It remains a fundamental equation in the field and is still widely used in various branches of physics and engineering.
Applications of Planck’s Radiation Formula
Planck’s radiation formula is a mathematical equation that describes the distribution of energy for electromagnetic radiation at different wavelengths emitted by a black body. This formula has several applications in various fields of science and technology. Some of the notable applications of Planck’s radiation formula include:
1. Astrophysics and Cosmology: Planck’s radiation formula is widely used in astrophysics to study the emission of radiation from astronomical objects such as stars, galaxies, and quasars. By applying this formula, scientists can extract information about the temperature and composition of these celestial bodies.
2. Infrared Thermography: Infrared thermography uses the principles of Planck’s radiation formula to measure infrared radiation emitted by objects. This technique is commonly used in industrial applications, such as monitoring the temperature of machinery, detecting energy loss in buildings, and detecting hidden defects in materials.
3. Climate Science: Planck’s radiation formula is an essential component of climate models used to study the Earth’s energy balance and climate change. It helps scientists understand the interaction between incoming solar radiation, outgoing terrestrial radiation, and greenhouse gases in the atmosphere.
4. Quantum Mechanics: Planck’s radiation formula played a crucial role in the development of quantum mechanics. It provided evidence for the quantization of energy, which ultimately led to the revolutionary understanding of energy levels in atomic and molecular systems.
5. Design of Lighting Systems: Planck’s radiation formula is used in lighting technology to design artificial lighting systems that mimic the spectral distribution of natural light sources. This formula helps determine the appropriate wavelengths and intensities of light required for different applications, such as indoor lighting, photography, and horticulture.
6. Radiation Therapy: In medical applications, Planck’s radiation formula is employed to calculate the energy distribution of ionizing radiation used in radiation therapy for cancer treatment. Understanding the radiation spectrum enables precise delivery of therapeutic radiation to target cancer cells while minimizing damage to healthy tissue.
These are just a few examples of the many applications of Planck’s radiation formula. Its mathematical formulation provides a fundamental understanding of the behavior of electromagnetic radiation, which has wide-ranging implications across various scientific and technological disciplines.
Conclusion
In conclusion, Planck’s radiation formula revolutionized the field of quantum mechanics by providing a mathematical description of the radiation emitted by a black body. The formula was developed by Max Planck in 1900 and is based on the concept of quantized energy levels, which was a departure from classical physics. Planck’s radiation formula accurately predicts the distribution of radiation at different wavelengths and temperatures, and it played a significant role in the development of quantum theory.
Moving forward, the use of Planck’s radiation formula continues to be invaluable in various fields, such as astrophysics, particle physics, and engineering. It provides a basis for understanding the behavior of electromagnetic radiation and is used in the development of technologies like lasers and detectors.
To further explore the implications of Planck’s radiation formula, future research can focus on applications in areas like energy production and quantum computing. Additionally, efforts can be made to refine and expand the formula to include more complex scenarios, such as different types of materials or non-equilibrium systems. Overall, understanding and continuing to study Planck’s radiation formula will contribute to the advancement of our knowledge and applications of quantum mechanics.
Topics related to Planckʼs Radiation Formula
planck's constant – YouTube
planck's constant – YouTube
Quantization of Energy Part 1: Blackbody Radiation and the Ultraviolet Catastrophe – YouTube
Quantization of Energy Part 1: Blackbody Radiation and the Ultraviolet Catastrophe – YouTube
DIMENSIONAL FORMULA OF PLANCK'S CONSTANT 🔥 #physics #shorts #dimensionalformula #dimensions – YouTube
DIMENSIONAL FORMULA OF PLANCK'S CONSTANT 🔥 #physics #shorts #dimensionalformula #dimensions – YouTube
Planck Radiation Law – A Quantum approach – YouTube
Planck Radiation Law – A Quantum approach – YouTube
Planck's Constant and BlackBody Radiation – YouTube
Planck's Constant and BlackBody Radiation – YouTube
Deriving Planck's Law | The Equation That Began Quantum Physics – YouTube
Deriving Planck's Law | The Equation That Began Quantum Physics – YouTube
planck's law of radiation in terms of wavelength। physics numerical questions। formula sheets। NDA। – YouTube
planck's law of radiation in terms of wavelength। physics numerical questions। formula sheets। NDA। – YouTube
in black body radiation for distribution of energy radiated by black body planck's formula reduces। – YouTube
in black body radiation for distribution of energy radiated by black body planck's formula reduces। – YouTube
Derivation of Planck's Radiation Law – YouTube
Derivation of Planck's Radiation Law – YouTube
Planck's Radiation Formula reduces to Wien's Formula and Rayleigh's Formula – YouTube
Planck's Radiation Formula reduces to Wien's Formula and Rayleigh's Formula – YouTube

Konstantin Sergeevich Novoselov is a Russian-British physicist born on August 23, 1974. Novoselov is best known for his groundbreaking work in the field of condensed matter physics and, in particular, for his co-discovery of graphene. Novoselov awarded the Nobel Prize in Physics. Konstantin Novoselov has continued his research in physics and materials science, contributing to the exploration of graphene’s properties and potential applications.