Introduction to the Carnot Cycle
The Carnot Cycle refers to a theoretical thermodynamic cycle that serves as a model for how heat engines, such as steam engines or internal combustion engines, operate. It was developed by French engineer Sadi Carnot in 1824 and is considered one of the most efficient cycles for converting thermal energy into mechanical work.
The Carnot Cycle consists of four stages:
1. Reversible isothermal expansion: In this stage, the working substance (usually a gas) absorbs heat from a high-temperature reservoir and expands while its temperature remains constant.
2. Reversible adiabatic expansion: The working substance continues to expand without any heat exchange, resulting in a decrease in its temperature and pressure.
3. Reversible isothermal compression: Heat is released from the working substance to a low-temperature reservoir while it is compressed, keeping its temperature constant.
4. Reversible adiabatic compression: The working substance is further compressed without any heat exchange, resulting in an increase in temperature and pressure.
The Carnot Cycle is unique because it is a theoretical cycle that maximizes the efficiency of converting heat into work. The efficiency of the cycle is determined by the temperature difference between the high-temperature and low-temperature reservoirs. The greater the temperature difference, the higher the efficiency.
The Carnot Cycle is used as a benchmark to compare the performance of different heat engines. Any real-world engine will have lower efficiency compared to the Carnot Cycle due to various factors such as friction, heat loss, and irreversibilities.
Despite being an idealized cycle, the Carnot Cycle provides a valuable theoretical foundation for the study of thermodynamics and serves as a guide for designing more efficient heat engines. It highlights the importance of operating at high temperatures and minimizing energy losses to improve the overall efficiency of engines.
Theoretical Description of the Carnot Cycle
The Carnot cycle is a theoretical cycle that describes the maximum efficiency possible for a heat engine operating between two temperature reservoirs. It was developed by French engineer Sadi Carnot in the 19th century and serves as a benchmark for evaluating the performance of real-life heat engines.
The Carnot cycle consists of four processes: two isothermal (constant temperature) processes and two adiabatic (no heat transfer) processes. It is typically represented on a pressure-volume (P-V) diagram.
The first process is an isothermal expansion at a high temperature. The working substance, which could be a gas or a fluid, absorbs heat from the high-temperature reservoir, causing its volume and pressure to increase while its temperature remains constant. This process is represented as an upward curve on the P-V diagram.
The second process is an adiabatic expansion. During this process, the working substance continues to expand, but without any heat transfer. As a result, its temperature and pressure decrease. This process is represented as a curved downward line on the P-V diagram.
The third process is an isothermal compression at a low temperature. The working substance is now brought in contact with the low-temperature reservoir, causing it to release heat and decrease in volume and pressure while its temperature remains constant. This process is represented as a downward curve on the P-V diagram.
The fourth and final process is an adiabatic compression. The working substance is compressed without any heat transfer, causing its temperature and pressure to increase. This process is represented as a curved upward line on the P-V diagram.
At the end of the cycle, the working substance returns to its initial state, and the cycle can start again.
The Carnot cycle is reversible, meaning it can be run backward by reversing the direction of each process. In this case, it becomes a refrigeration cycle, where heat is transferred from a low-temperature reservoir to a high-temperature reservoir.
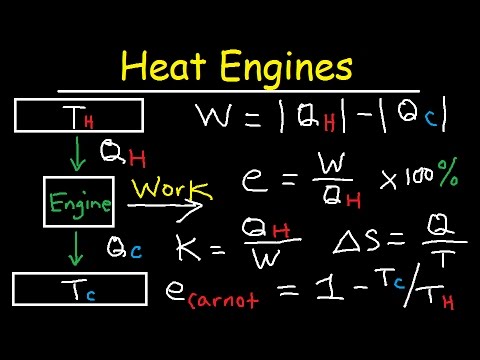
The efficiency of the Carnot cycle is determined by the temperatures of the high and low temperature reservoirs. The maximum efficiency can be calculated using the Carnot efficiency formula, which states that the efficiency is equal to 1 minus the ratio of the absolute temperatures of the reservoirs.
While the Carnot cycle is an idealized model that does not occur in real-life engines, it serves as a useful tool for comparing and evaluating the performance of practical heat engines.
Efficiency of the Carnot Cycle
The Carnot cycle is known for its high efficiency and is often considered to be the most efficient heat engine cycle possible. It operates in a reversible manner, making it ideal for theoretical calculations.
The efficiency of the Carnot cycle is given by the equation:
Efficiency = 1 – (Tc/Th)
where Tc is the temperature of the cold reservoir and Th is the temperature of the hot reservoir.
The Carnot cycle achieves its maximum efficiency when operating between the highest and lowest temperatures possible. As the temperature difference increases, the efficiency also increases.
However, in practice, it is impossible to achieve the conditions necessary for a perfect Carnot cycle, such as isothermal and adiabatic processes. Real-world engines experience losses and inefficiencies due to factors like friction, heat transfer, and internal energy losses. Therefore, the actual efficiency of practical heat engines is always lower than the maximum efficiency that the Carnot cycle can achieve.
Limitations and Applications of the Carnot Cycle
The Carnot cycle is a theoretical thermodynamic cycle that is used to analyze the maximum efficiency that a heat engine can achieve. It is made up of four reversible processes: isothermal heat addition, adiabatic expansion, isothermal heat rejection, and adiabatic compression. The limitations and applications of the Carnot cycle are as follows:
1. Limitations:
a. Idealized conditions: The Carnot cycle assumes ideal conditions, such as no friction, perfect insulation, and reversible processes. However, in real-world applications, these ideal conditions are difficult, if not impossible, to achieve.
b. Requires a perfect gas: The Carnot cycle assumes the working fluid is a perfect gas. Real gases may deviate from ideal gas behavior, leading to inefficiencies and deviations from the Carnot cycle predictions.
c. Specific temperature range: The efficiency of the Carnot cycle is directly related to the temperature difference between the hot and cold reservoirs. If the temperature difference is small, the efficiency will also be low.
d. Slow process: The Carnot cycle requires slow and gradual heat exchange processes. This makes it impractical for applications where high power output is required, such as in modern power plants.
2. Applications:
a. Benchmark for efficiency: The Carnot cycle serves as a benchmark for comparing the efficiencies of real heat engines. It allows engineers to evaluate how close a real engine can come to the maximum theoretical efficiency.
b. Design and optimization of heat engines: The Carnot cycle provides insights into the optimal design of heat engines. By studying the Carnot cycle, engineers can identify areas for improvement and develop strategies to increase the efficiency of real engines.
c. Thermodynamic analysis: The Carnot cycle is a fundamental concept in thermodynamics and helps in understanding key principles, such as reversibility, work, and heat transfer. It forms the basis for the development of other thermodynamic cycles, such as the Rankine cycle for power generation and the refrigeration cycle for cooling systems.
d. Educational tool: The Carnot cycle is used as a teaching tool in thermodynamics and engineering courses. It helps students understand the concepts of heat engines, efficiency, and the limitations of real-world systems.
In summary, while the Carnot cycle has limitations due to its idealized assumptions, it is a valuable tool for analyzing and understanding the efficiency of heat engines. It serves as a benchmark for comparisons, aids in the design and optimization of engines, and provides a foundation for thermodynamic analysis.
Conclusion
In conclusion, the Carnot Cycle is a theoretical thermodynamic cycle that represents the most efficient way to convert heat into work. It consists of four reversible processes – isothermal heat transfer, adiabatic expansion, isothermal heat rejection, and adiabatic compression. The Carnot Cycle operates between two temperature reservoirs, and its efficiency is determined solely by the temperatures of these reservoirs. Due to its idealized nature, the Carnot Cycle serves as a benchmark for comparison with real-world heat engines, helping to assess their performance and improve their efficiency.
Topics related to Carnot Cycle
Carnot Cycle & Heat Engines, Maximum Efficiency, & Energy Flow Diagrams Thermodynamics & Physics – YouTube
Carnot Cycle & Heat Engines, Maximum Efficiency, & Energy Flow Diagrams Thermodynamics & Physics – YouTube
CARNOT CYCLE | Easy and Basic – YouTube
CARNOT CYCLE | Easy and Basic – YouTube
Carnot Heat Engines, Efficiency, Refrigerators, Pumps, Entropy, Thermodynamics – Second Law, Physics – YouTube
Carnot Heat Engines, Efficiency, Refrigerators, Pumps, Entropy, Thermodynamics – Second Law, Physics – YouTube
Carnot cycle, Carnot – YouTube
Carnot cycle, Carnot – YouTube
Carnot cycle|P-V diagram| #ncert #carnotcycle #tricks #fun – YouTube
Carnot cycle|P-V diagram| #ncert #carnotcycle #tricks #fun – YouTube
Carnot heat engine… Carnot cycle.. Carnot cycle construction… bsc physics important topic – YouTube
Carnot heat engine… Carnot cycle.. Carnot cycle construction… bsc physics important topic – YouTube
Carnot engine/ efficiency/ JEE MAIN 2022- Vivek Phalke PHYSICS – YouTube
Carnot engine/ efficiency/ JEE MAIN 2022- Vivek Phalke PHYSICS – YouTube
4-Stroke (Four Stroke) Engine (Carnot Engine) Homemade Model Project for class 11 students….|| – YouTube
4-Stroke (Four Stroke) Engine (Carnot Engine) Homemade Model Project for class 11 students….|| – YouTube
working of carnot engine – YouTube
working of carnot engine – YouTube
Carnot cycle explain – YouTube
Carnot cycle explain – YouTube

Konstantin Sergeevich Novoselov is a Russian-British physicist born on August 23, 1974. Novoselov is best known for his groundbreaking work in the field of condensed matter physics and, in particular, for his co-discovery of graphene. Novoselov awarded the Nobel Prize in Physics. Konstantin Novoselov has continued his research in physics and materials science, contributing to the exploration of graphene’s properties and potential applications.