Introduction to De Broglie Wavelength
The De Broglie wavelength, named after the French physicist Louis de Broglie, refers to the wave-like nature of matter particles. It was a groundbreaking idea proposed by de Broglie in 1924, which suggested that all particles, including electrons, protons, and atoms, could exhibit wave-like behavior.
De Broglie’s concept can be understood by considering the dual nature of light. Just as light can act as both a particle and a wave, de Broglie propose that particles also have wave properties. This implies that matter particles, despite their tininess and mass, can carry a wave character.
The wavelength associated with a particle, known as the De Broglie wavelength, is determined by its momentum. The greater the momentum, the shorter the wavelength. This concept is interconnected with the principle of quantum mechanics, which describes the behavior of particles on the atomic and subatomic level.
The De Broglie wavelength has been experimentally verified through various techniques, such as electron diffraction and interference experiments. These experiments have demonstrated that particles, even those commonly considered as solid objects, can show wave-like behavior and exhibit interference patterns similar to those observed in light wave experiments.
The De Broglie wavelength has profound implications in our understanding of quantum mechanics and the behavior of particles at the microscopic level. It provides a deeper insight into the fundamental nature of matter and has paved the way for numerous advancements, including electron microscopy and the development of quantum computing.
Theoretical Background of De Broglie Wavelength
The De Broglie wavelength is a concept in quantum mechanics that describes the wave-like behavior of matter particles such as electrons and protons. It was introduced by Louis de Broglie in 1924 as part of his theory of wave-particle duality.
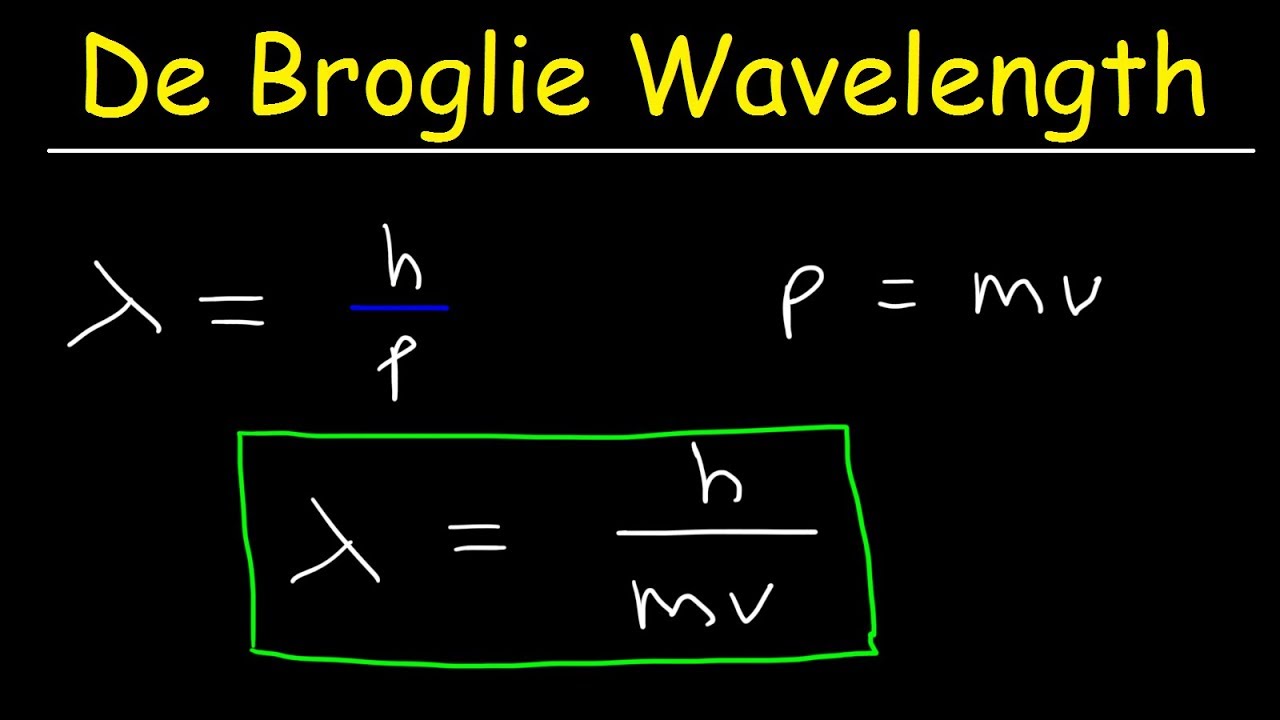
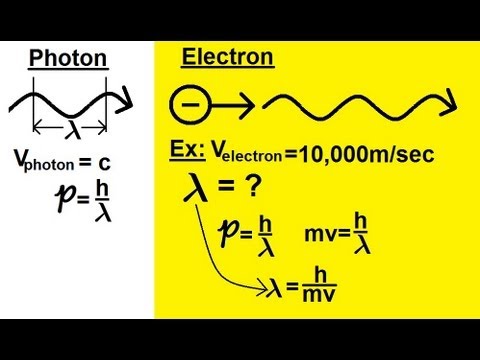
According to de Broglie’s hypothesis, every particle with momentum p can also exhibit wave-like properties with a corresponding wavelength λ, which is known as the de Broglie wavelength. This wavelength is related to the momentum of the particle through the equation λ = h/p, where h is the Planck constant.
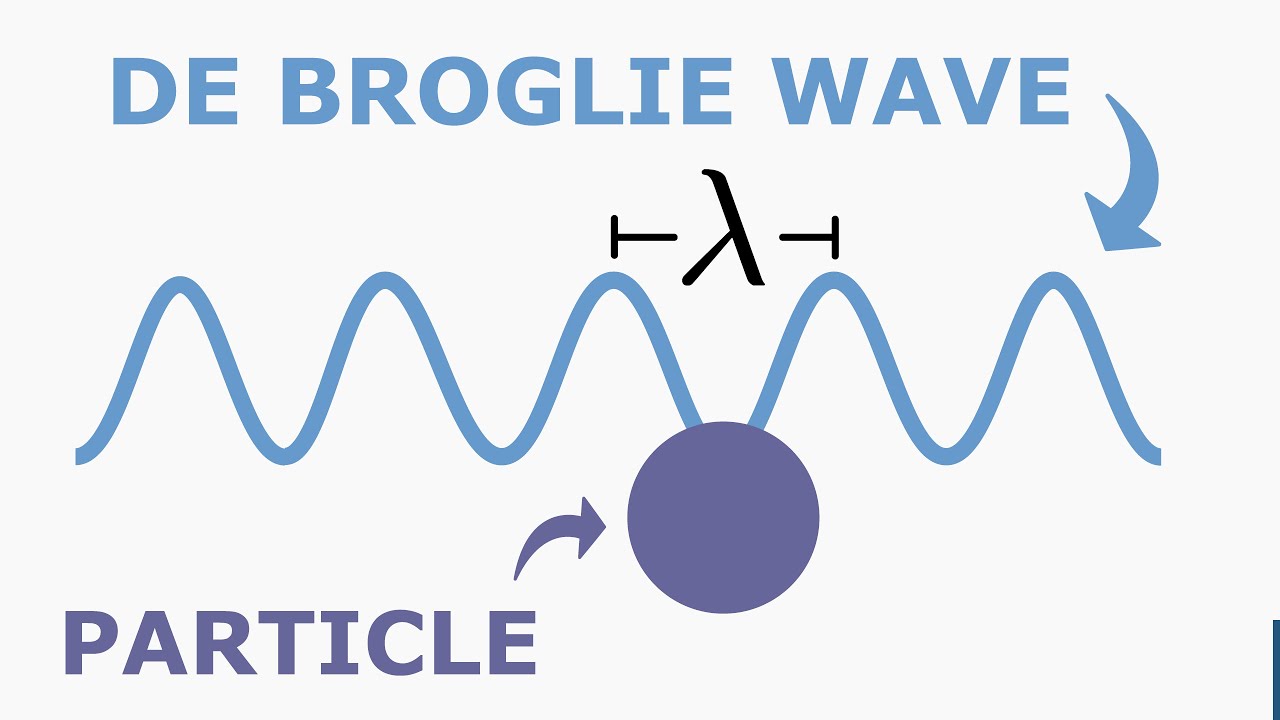
The de Broglie wavelength can be thought of as the characteristic length scale associated with a particle’s wave-like behavior. It represents the spatial extent of the probability distribution of the particle’s position, or in other words, the region in which the particle is most likely to be found.
One of the important implications of the de Broglie wavelength is that it provides a bridge between classical and quantum mechanics. In classical physics, particles are treated as point-like objects with definite positions and momenta. However, in quantum mechanics, particles are described by wavefunctions that can spread out in space. The de Broglie wavelength captures this wave-like nature of particles and allows us to calculate the probability of finding a particle at a particular position.
The de Broglie wavelength also has practical consequences in quantum mechanics. For example, it is used to explain phenomena such as electron diffraction and interference, where electrons exhibit wave-like behavior when passing through a narrow slit or interfering with each other. It also plays a crucial role in the understanding of quantum tunneling, where particles can pass through barriers that would be classically forbidden.
In summary, the de Broglie wavelength is a fundamental concept in quantum mechanics that describes the wave-like behavior of matter particles. It provides a link between classical and quantum physics and has important implications for our understanding of the behavior of particles at the microscopic level.
Applications of De Broglie Wavelength
The concept of De Broglie wavelength, derived from Louis de Broglie’s wavelength matter wave theory, has several applications in physics. Some of the main applications include:
1. Particle Diffraction: De Broglie wavelength provides a theoretical basis for the phenomenon of particle diffraction. Just as light waves diffract when passing through a narrow slit, particles also show diffraction patterns when passing through small openings. The De Broglie wavelength helps determine the extent of this diffraction and is used in experiments involving electron diffraction and neutron diffraction.
2. Electron Microscopy: Electron microscopy relies on the wave-like nature of electrons and their De Broglie wavelength. Since the De Broglie wavelength of electrons is much smaller than visible light, electron microscopes have higher resolution capabilities than optical microscopes. The shorter De Broglie wavelength allows for the visualization of atomic and molecular structures in greater detail.
3. Quantum Mechanics: De Broglie’s concept of matter waves was significant in the development of quantum mechanics. It provided a theoretical framework to describe the wave-particle duality of particles, including electrons, protons, and other subatomic particles. The De Broglie wavelength is an essential quantity in the mathematical representation of particle behavior in quantum mechanics.
4. Electron Tunneling: Electron tunneling is a quantum mechanical phenomenon where electrons can pass through potential barriers that would be classically forbidden. The probability of electron tunneling occurring depends on the energy of the electron and the width of the barrier, which can be described using the De Broglie wavelength.
5. Atomic Spectroscopy: The De Broglie wavelength is used in the analysis of atomic spectra. It helps determine the energy levels and transitions of electrons in atoms, which are responsible for the emission and absorption of light at specific wavelengths. By calculating the De Broglie wavelength, the behavior of electrons in atomic systems can be understood more accurately.
6. Quantum Computing: The De Broglie wavelength plays a role in understanding and manipulating quantum states in quantum computing. Quantum computers make use of the wave-particle duality of quantum systems, and the De Broglie wavelength helps determine the behavior and interactions of quantum bits (qubits).
Overall, the De Broglie wavelength is a fundamental concept that has advanced our understanding of the behavior of particles at the quantum level and has numerous applications in various fields of physics.
Experimental Verification of De Broglie Wavelength
De Broglie wavelength is a concept in quantum mechanics that suggests that particles such as electrons and protons can exhibit wave-like properties. It was proposed by Louis de Broglie in 1924 and later experimentally verified.
The experimental verification of De Broglie wavelength was first demonstrated by Clinton J. Davisson and Lester H. Germer in 1927. They performed a groundbreaking experiment known as the Davisson-Germer experiment, which confirmed the wave-particle duality of matter.
In their experiment, Davisson and Germer directed a beam of electrons onto a nickel crystal surface. They observed that the electrons were diffracted by the crystal, similar to the diffraction of waves by a grating. The diffraction pattern obtained on a screen behind the crystal demonstrated the wave-like behavior of the electrons.
The key finding of the experiment was that the diffraction pattern observed matched the predictions of the De Broglie wavelength equation, which relates the wavelength of a particle to its momentum. This confirmed that electrons indeed possess wave properties and that their behavior could be described using the De Broglie wavelength.
This experimental verification of the De Broglie wavelength provided crucial evidence for the wave-particle duality of matter and further supported the development of quantum mechanics. It also laid the foundation for the understanding of quantum phenomena and the wave nature of particles at the atomic and subatomic levels.
Implications and Significance of De Broglie Wavelength in Physics
The de Broglie wavelength, derived by physicist Louis de Broglie, is a concept that relates to the wave-particle duality of matter. It suggests that particles, such as electrons and other subatomic particles, can exhibit wave-like properties.
The implications and significance of the de Broglie wavelength in physics are numerous:
1. Wave-Particle Duality: The de Broglie wavelength provides experimental evidence for the wave-particle duality of matter. It shows that particles have both particle-like and wave-like properties. This concept challenged the classical understanding of particles as only discrete entities and paved the way for the development of quantum mechanics.
2. Uncertainty Principle: The de Broglie wavelength is related to the uncertainty principle proposed by Werner Heisenberg. According to the uncertainty principle, it is impossible to simultaneously determine both the position and momentum of a particle with absolute precision. The de Broglie wavelength is a fundamental factor in the uncertainty principle, as it determines the limit to which the position of a particle can be known.
3. Diffraction and Interference: Just like waves can diffract and interfere with each other, particles with a finite de Broglie wavelength can also exhibit these behaviors. This phenomenon has been experimentally confirmed with electrons and other subatomic particles. Diffraction and interference patterns caused by particles have been observed in experiments, demonstrating their wave-like nature.
4. Wave Nature of Electrons: The de Broglie wavelength is particularly significant for electrons, as it explains their behavior in atoms and solid-state physics. The wavelength of electrons in atomic orbitals determines the stability and energy levels of electrons in an atom, leading to the creation of the periodic table. Additionally, in solid-state physics, the de Broglie wavelength helps explain the behavior of electrons in crystal lattices and their contribution to properties like electrical conductivity and thermal resistance.
5. Particle Accelerators: The de Broglie wavelength has practical implications in particle accelerators. As particles are accelerated to high energies, their de Broglie wavelength becomes smaller, potentially allowing for more precise measurements and control of their behavior.
Overall, the de Broglie wavelength plays a crucial role in understanding the fundamental nature of matter and has significant implications for various areas of physics, including quantum mechanics, atomic physics, and solid-state physics. It provides a deep insight into the wave-particle duality and helps explain the behavior of particles in both macroscopic and microscopic systems.
Topics related to De Broglie Wavelength
De Broglie Wavelength Problems In Chemistry – YouTube
De Broglie Wavelength Problems In Chemistry – YouTube
The de Broglie Wavelength and Wave Particle Duality – A Level Physics – YouTube
The de Broglie Wavelength and Wave Particle Duality – A Level Physics – YouTube
De-Broglie Wavelength – YouTube
De-Broglie Wavelength – YouTube
de Broglie wavelength in different frames – YouTube
de Broglie wavelength in different frames – YouTube
Physics – Modern Physics (11 of 26) The de Broglie Wavelength – YouTube
Physics – Modern Physics (11 of 26) The de Broglie Wavelength – YouTube
De Broglie Hypothesis | De Broglie Wavelength – YouTube
De Broglie Hypothesis | De Broglie Wavelength – YouTube
de Broglie’s proposal – YouTube
de Broglie’s proposal – YouTube
Electron Diffraction – A-level Physics – YouTube
Electron Diffraction – A-level Physics – YouTube
Wave Particle Duality – Basic Introduction – YouTube
Wave Particle Duality – Basic Introduction – YouTube
The photoelectric effect – YouTube
The photoelectric effect – YouTube

Konstantin Sergeevich Novoselov is a Russian-British physicist born on August 23, 1974. Novoselov is best known for his groundbreaking work in the field of condensed matter physics and, in particular, for his co-discovery of graphene. Novoselov awarded the Nobel Prize in Physics. Konstantin Novoselov has continued his research in physics and materials science, contributing to the exploration of graphene’s properties and potential applications.