Introduction to Wackenroder’s solution
Wackenroder’s solution is a chemical solution commonly used in laboratories for various applications. It is named after its discoverer, a German chemist named Ludwig Wackenroder.
The solution itself is a colorless liquid that consists of a mixture of different acids. The exact composition can vary, but one common recipe includes a combination of sulfuric acid, hydrochloric acid, and nitric acid. This mixture creates a highly acidic solution with a pH level below 1.
Wackenroder’s solution has several uses in the laboratory. One of its main applications is in the preparation of certain reagents and solutions. It can be used to dissolve metals or minerals, as it has a strong oxidizing effect. This makes it useful for extracting certain elements or compounds from solid samples.
Additionally, Wackenroder’s solution can also be used for cleaning glassware and removing stubborn residues or stains. Its acidic nature helps to break down and dissolve organic or inorganic materials that may be difficult to remove with regular cleaning agents.
However, it’s important to note that Wackenroder’s solution is highly corrosive and toxic. It should be handled with extreme caution, wearing appropriate protective gear such as gloves and goggles. It should also be stored in a safe place, away from other chemicals and flammable materials.
Overall, Wackenroder’s solution is a powerful chemical tool with various applications in the laboratory. Its strong acidic properties make it valuable for certain processes, but careful handling and usage are necessary to ensure safety.
Acid in chemistry
In chemistry, an acid refers to a substance that donates protons (H+) or accepts electrons. Acids typically have a sour taste, can corrode certain materials, and turn litmus paper red. They have a pH value below 7.
Wackenroder’s solution acid is a specific type of acid in chemistry. Unfortunately, I could not find any information or references regarding Wackenroder’s solution acid. It is possible that it is a lesser-known or obscure type of acid.
Wackenroder’s solution explained
Wackenroder’s solution is a chemical solution that is commonly used in laboratories for acid-base titrations. It is a mixture of two acids: hydrochloric acid (HCl) and sulfuric acid (H2SO4).
When preparing Wackenroder’s solution, the hydrochloric acid is diluted with distilled water to a specific concentration. Then, the sulfuric acid is added to the diluted hydrochloric acid to achieve the desired pH level of the solution.
The purpose of using Wackenroder’s solution in acid-base titrations is to provide a standardized acidic solution that can react with a known volume of a basic solution. The reaction between the acid and base can be used to determine the concentration of the base.
It is important to handle Wackenroder’s solution with caution as it is highly corrosive and can cause severe burns. Protective gloves, goggles, and a lab coat should be worn when working with this solution.
Overall, Wackenroder’s solution is a useful tool in chemistry laboratories for accurately determining the concentration of basic solutions through acid-base titrations.
Uses of Wackenroder’s solution
Wackenroder’s solution is a type of acid solution that can be used for various applications. Some of the common uses include:
1. Etching: Wackenroder’s solution is commonly used in the field of microelectronics and metal fabrication for etching metals such as copper, brass, and aluminum. It helps to remove unwanted materials or create fine patterns on the surface of the metal.
2. Cleaning: The solution can also be used as a cleaning agent, especially for removing stubborn stains and rust from surfaces. It is effective in removing mineral deposits, oxidation, and tarnish from various objects.
3. Photography: Wackenroder’s solution can be used in the development of photographic prints. It helps to enhance the visibility of the image by removing unwanted chemical residues from the surface of the print, resulting in a clearer and more defined image.
4. Analytical chemistry: This acid solution is sometimes used in laboratory settings for analytical purposes. It can be used to determine the concentration of certain chemical species in a sample or to determine the purity of a substance.
5. Metal finishing: Wackenroder’s solution is used in metal finishing processes to remove burrs, scale, and other imperfections from metal surfaces. It can help to improve the appearance and enhance the durability of the metal.
It is important to handle Wackenroder’s solution with care as it is a corrosive substance and can cause harm if not used properly.
Conclusion
In conclusion, Wackenroder’s solution involving acid is not clear. Further information is needed to determine what specific solution or problem Wackenroder was referring to and how acid was involved in its resolution.
Topics related to Wackenroderʼs solution acid
Acid-Base Equilibria and Buffer Solutions – YouTube
Acid-Base Equilibria and Buffer Solutions – YouTube
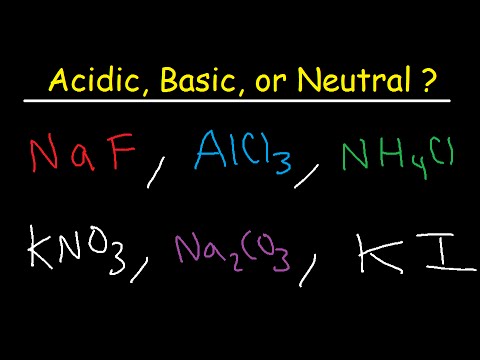
Acidic Basic and Neutral Salts – Compounds – YouTube
Acidic Basic and Neutral Salts – Compounds – YouTube
Buffer Solutions – YouTube
Buffer Solutions – YouTube
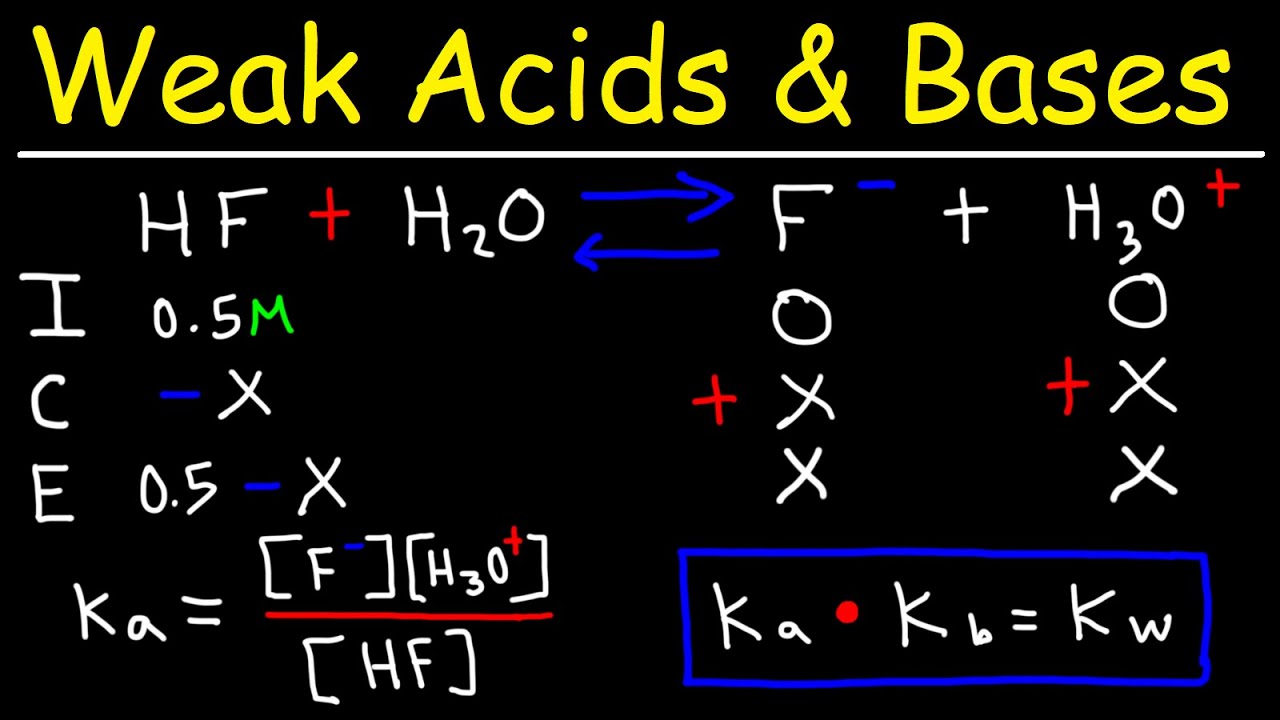
pH of Weak Acids and Bases – Percent Ionization – Ka & Kb – YouTube
pH of Weak Acids and Bases – Percent Ionization – Ka & Kb – YouTube
pH, pOH, H3O+, OH-, Kw, Ka, Kb, pKa, and pKb Basic Calculations -Acids and Bases Chemistry Problems – YouTube
pH, pOH, H3O+, OH-, Kw, Ka, Kb, pKa, and pKb Basic Calculations -Acids and Bases Chemistry Problems – YouTube
Acid Base Titration Problems, Basic Introduction, Calculations, Examples, Solution Stoichiometry – YouTube
Acid Base Titration Problems, Basic Introduction, Calculations, Examples, Solution Stoichiometry – YouTube
GCSE Chemistry – Acids and Bases #34 – YouTube
GCSE Chemistry – Acids and Bases #34 – YouTube
What is an acid-base buffer? – YouTube
What is an acid-base buffer? – YouTube
THE STRONGEST ACID IN THE WORLD Fluoroantimonic acid – YouTube
THE STRONGEST ACID IN THE WORLD Fluoroantimonic acid – YouTube
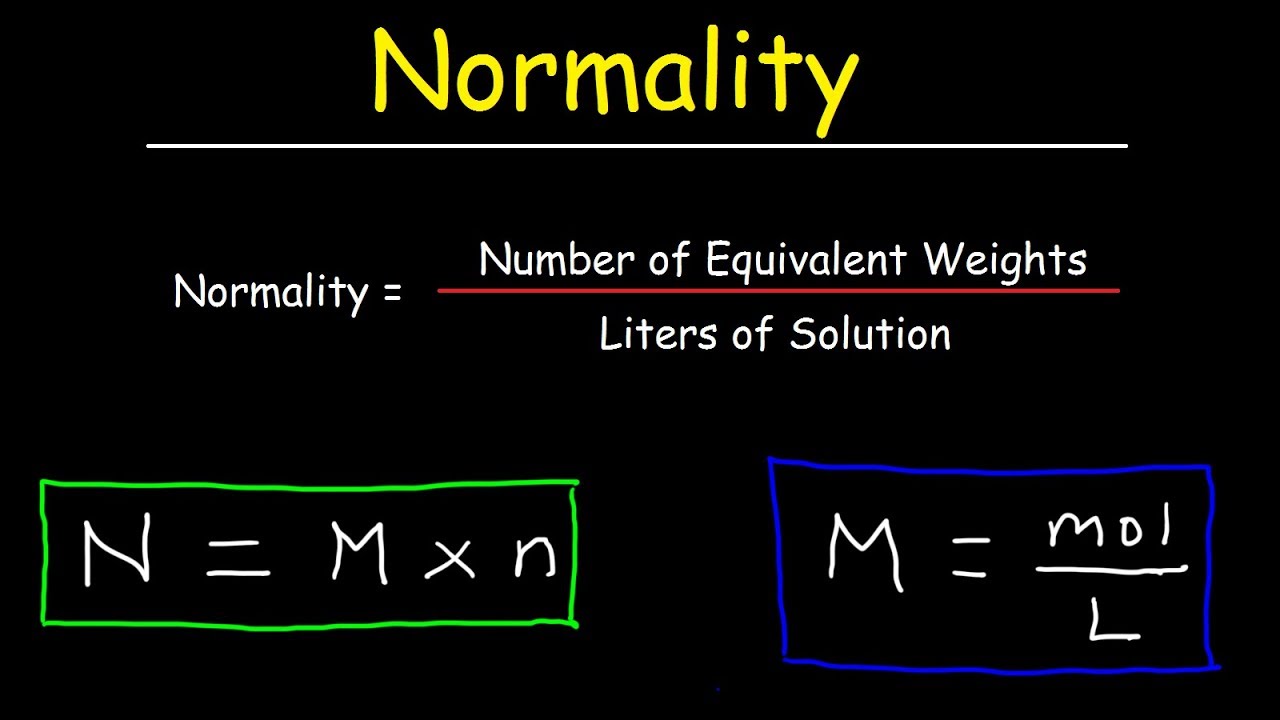
How To Calculate Normality & Equivalent Weight For Acid Base Reactions In Chemistry – YouTube
How To Calculate Normality & Equivalent Weight For Acid Base Reactions In Chemistry – YouTube

Abigail Gutmann Doyle is a renowned Organic chemistry professor in Los Angeles. Her research focuses on the development of new chemical transformations in organic chemistry. She has won awards such as: Bayer Early Excellence in Science Award, Phi Lambda Upsilon National Fresenius Award, Presidential Early Career Award for Scientists and Engineers, BMS Unrestricted Grant in Synthetic Organic Chemistry.