Definition of Ammonium Hydroxide
Ammonium hydroxide is a chemical compound with the formula NH4OH. It is also commonly referred to as ammonia solution or aqueous ammonia. Ammonium hydroxide is a solution of ammonia (NH3) gas dissolved in water (H2O). It is a colorless liquid with a pungent odor and is commonly used in various industrial and household applications.
Ammonium hydroxide is a weak base, and it can react with acids to form ammonium salts. It is often used as a cleaning agent, as it can help remove dirt, grease, and stains from various surfaces. In addition to cleaning, it is employed in various chemical processes, including as a reagent in laboratories and as a pH adjuster in various applications. When handling ammonium hydroxide, it is important to take appropriate safety precautions due to its potential for causing irritation and harm to the skin, eyes, and respiratory system.
Chemical Formula and Structure
The chemical formula for ammonium hydroxide is NH4OH. However, it’s important to note that NH4OH is a simplification and does not accurately represent the actual chemical structure of the compound.
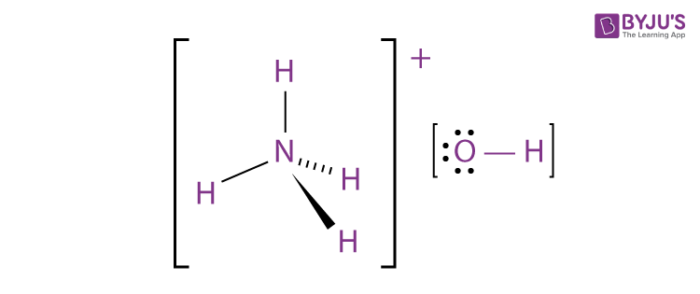
The structure of ammonium hydroxide is more accurately described as a solution of ammonia (NH3) dissolved in water (H2O). Ammonia is a covalent compound consisting of one nitrogen atom (N) bonded to three hydrogen atoms (H), and water is composed of two hydrogen atoms (H) and one oxygen atom (O) bonded together. When ammonia dissolves in water, it forms ammonium ions (NH4+) and hydroxide ions (OH-) due to the interaction between the ammonia molecules and water molecules. This is a result of ammonia’s ability to act as a weak base and accept a proton (H+) from water, forming NH4+ and OH- ions.
So, the structure of ammonium hydroxide in solution can be represented as follows:
NH3 + H2O → NH4+ + OH-
In this representation, NH4+ is the ammonium ion, and OH- is the hydroxide ion. These ions are responsible for the basic properties of the solution.
Properties of Ammonium Hydroxide
Ammonium hydroxide, or ammonia solution, has several properties that are important to its various applications and handling. Here are some key properties of ammonium hydroxide:
Appearance: Ammonium hydroxide is a colorless liquid. It may have a slightly cloudy or milky appearance due to the presence of dissolved ammonia gas.
Odor: It has a pungent and distinct ammonia-like odor, which can be quite strong and irritating at higher concentrations.
pH: Ammonium hydroxide is a weak base and can raise the pH of a solution. It typically has a pH in the range of 10 to 11 when used at typical concentrations. The pH may vary depending on the concentration and purity of the solution.
Solubility: Ammonium hydroxide is highly soluble in water, and it readily dissolves in water to form ammonium ions (NH4+) and hydroxide ions (OH-).
Chemical Reactivity: It can react with acids to form ammonium salts. It is often used as a neutralizing agent in chemical processes to adjust the pH.
Cleaning Properties: Ammonium hydroxide is used as a cleaning agent due to its ability to dissolve and remove dirt, grease, and stains from various surfaces, including glass and metals.
Vapor Pressure: It can release ammonia gas into the air, which contributes to its strong odor. This can be a safety concern in enclosed spaces.
Corrosivity: Concentrated ammonium hydroxide solutions can be corrosive to certain materials, such as aluminum, and can cause damage to some surfaces over time.
Toxicity: Ammonium hydroxide can be harmful when inhaled or when it comes into contact with the skin or eyes. Proper safety precautions and protective equipment should be used when handling it.
Storage and Handling: It should be stored in well-ventilated areas and kept away from incompatible substances, especially strong acids. Proper storage and handling procedures should be followed to ensure safety.
Concentrations: Ammonium hydroxide solutions are available in various concentrations, typically ranging from 5% to 30%. The concentration can affect its reactivity and properties.
It’s important to note that the specific properties of ammonium hydroxide can vary depending on its concentration and purity, so care should be taken when using this chemical in various applications, and safety guidelines should be followed to minimize risks associated with its use.
Uses of Ammonium Hydroxide in Chemistry
Ammonium hydroxide (NH4OH) is a commonly used compound in various chemical processes. Here are some of its uses in chemistry:
1. Titration: It is often used as a titrant in acid-base titrations to determine the concentration of acids or bases in a solution. It reacts with acids to form ammonium salts, while reacting with bases to form ammonia and water.
2. pH adjustment: Ammonium hydroxide is used to adjust the pH in various chemical reactions. It can be added to acidic solutions to raise the pH and make them more alkaline.
3. Solvent: It is used as a solvent for many organic and inorganic compounds in analytical chemistry. It can dissolve a wide range of substances and is often used for extraction and purification processes.
4. Precipitation of metal hydroxides: Ammonium hydroxide is used to precipitate certain metal hydroxides from solutions. When added to a metal salt solution, it forms insoluble metal hydroxides which can be collected and further processed.
5. Buffer solutions: Ammonium hydroxide can act as a buffer solution when combined with a weak acid such as ammonium chloride (NH4Cl). These buffer solutions help maintain a stable pH when small amounts of acid or base are added.
6. Textile and paper industries: Ammonium hydroxide is used in the textile and paper industries as a stabilizer, pH adjuster, and as a bleaching and dyeing agent.
7. Cleaning agent: It is commonly found in household cleaning products such as glass cleaners, floor cleaners, and bathroom cleaners. It acts as a powerful cleaner and removes dirt, grease, and stains from surfaces.
8. Inorganic analysis: Ammonium hydroxide is used in qualitative and quantitative analysis of various elements and compounds. It helps in the separation and identification of different ions and compounds in analytical chemistry.
9. Fertilizers: Ammonium hydroxide can be used to produce ammonium-based fertilizers, as it provides a readily available source of nitrogen, one of the essential elements for plant growth.
These are just some of the many uses of ammonium hydroxide in chemistry. It is a versatile compound that finds applications in various fields of chemical research, industry, and everyday life.
Safety and Precautions
When working in a chemistry lab or conducting chemical experiments, it is important to follow safety precautions to minimize the risk of accidents and injuries. Here are some general safety guidelines to keep in mind:
1. Familiarize yourself with the lab: Read and understand the lab safety rules and procedures specific to your laboratory. Know the locations of safety equipment such as fire extinguishers, eyewash stations, safety showers, and fire blankets.
2. Personal Protective Equipment (PPE): Always wear appropriate PPE, including a lab coat or chemical-resistant apron, safety goggles or a face shield, and gloves when handling chemicals. Depending on the experiment, additional PPE such as closed-toe shoes, long pants, and a respirator may be necessary.
3. Chemical handling and storage: Follow proper procedures for handling, storage, and disposal of chemicals. Always label chemical containers appropriately, and keep incompatible chemicals separate to prevent reactions. Use a fume hood when working with volatile or toxic chemicals.
4. Ventilation: Ensure that your work area is adequately ventilated. Use fume hoods when working with volatile or hazardous chemicals to minimize exposure to vapors and fumes.
5. Fire safety: Know the location of fire extinguishers in your lab and how to use them. Practice good housekeeping by keeping your workspace clean and free of excess clutter. Avoid using open flames near flammable substances and be cautious when using heat sources.
6. Emergency procedures: Familiarize yourself with the emergency procedures specific to your lab, including how to evacuate, where to assemble, and whom to notify in case of an accident or emergency.
7. Electrical safety: Inspect electrical equipment before use, and never use damaged or frayed electrical cords. Be cautious when working with electrical equipment near water or when handling wet hands. Use grounded outlets and ensure proper grounding of equipment.
8. Use appropriate tools: Use the right tools and equipment for each experiment or task. Improper tools can lead to accidents or dangerous situations.
9. Personal hygiene: Avoid eating, drinking, or applying cosmetics in the lab. Wash your hands thoroughly with soap and water after working with chemicals, and avoid touching your face or eyes.
10. Be prepared for spills and accidents: Have spill kits and absorbent materials readily available in the lab to clean up spills promptly. Know how to safely handle and dispose of broken glassware. Report any accidents, spills, or near misses to the appropriate personnel.
Remember, safety should always be a priority in the chemistry lab. Following these precautions will help protect yourself, your colleagues, and the environment from potential hazards and accidents.

Abigail Gutmann Doyle is a renowned Organic chemistry professor in Los Angeles. Her research focuses on the development of new chemical transformations in organic chemistry. She has won awards such as: Bayer Early Excellence in Science Award, Phi Lambda Upsilon National Fresenius Award, Presidential Early Career Award for Scientists and Engineers, BMS Unrestricted Grant in Synthetic Organic Chemistry.