Introduction
Ernest Rutherford is credited with developing the nuclear model of the atom, which revolutionized our understanding of atomic structure. His model built upon the previous work of J.J. Thomson and others, who discovered the existence of subatomic particles known as electrons. However, Rutherford’s experiments led him to propose a new model that explained the distribution of positive charge within the atom.
Rutherford’s experiments involved firing alpha particles (positively charged particles) at a thin sheet of gold foil. He expected the particles to pass through the foil with minor deflections, as was predicted by the prevailing “plum pudding” model of the atom. However, to his surprise, some of the particles were deflected at large angles and even bounced back. This observation could not be explained by the plum pudding model.
Based on these findings, Rutherford hypothesized that the atom consists of a small, dense, positively charged nucleus at the center, with electrons orbiting around it. He proposed that most of the atom’s mass is concentrated in the nucleus, while the electrons occupy a relatively large, empty space surrounding it.
Rutherford’s nuclear model of the atom had significant implications. It explained the unexpected deflections of the alpha particles and revealed that the atom is mostly empty space. The model also provided a new understanding of atomic stability and the nature of nuclear reactions.
However, Rutherford’s model did not fully address certain observations, such as the distribution of electrons within the atom and their behavior. Subsequent advancements in quantum mechanics would lead to the development of more accurate models of atomic structure. Nonetheless, Rutherford’s nuclear model was a significant milestone in the history of atomic theory and laid the foundation for further exploration in the field.
Rutherford’s Nuclear Model
Rutherford’s nuclear model, also known as the Rutherford model, is a model of the atom proposed by New Zealand physicist Ernest Rutherford in 1911. This model is based on his famous gold foil experiment, in which he directed a beam of alpha particles at a thin sheet of gold foil and observed their scattering patterns.
According to Rutherford’s model, atoms consist of a tiny, positively charged nucleus at the center, surrounded by negatively charged electrons in orbits or shells. The nucleus is extremely dense and contains most of the atom’s mass, while the electrons are much smaller and occupy most of the volume of the atom.
In the gold foil experiment, Rutherford discovered that most of the alpha particles passed straight through the foil with little or no deflection, while a small fraction were deflected at various angles and some even bounced back. This led him to conclude that the atom’s positive charge and most of its mass were concentrated in a small, dense nucleus, with the electrons occupying a larger, relatively empty space around it.
Rutherford’s model revolutionized the understanding of atomic structure and led to the development of the modern understanding of the atom. However, it was later refined by the Bohr model, and further advancements in quantum mechanics led to the development of the current model known as the electron cloud model. Nonetheless, Rutherford’s nuclear model laid the foundation for future discoveries and understanding of atomic physics.
Structure of the Atom
The structure of the atom refers to its basic composition and organization. In 1911, Ernest Rutherford proposed the nuclear model of the atom, which revolutionized our understanding of atomic structure.
According to Rutherford’s model, atoms consist of a tiny, dense nucleus located at the center. The nucleus is positively charged and contains most of the atom’s mass. Surrounding the nucleus are negatively charged electrons, which are much lighter and move in orbits or energy levels around the nucleus.
The nucleus is composed of positively charged particles called protons and uncharged particles called neutrons. The number of protons in the nucleus determines the element’s atomic number and gives it its unique identity. Neutrons, on the other hand, contribute to the mass of the atom but not its charge.
The electrons exist in different energy shells or orbitals around the nucleus. These shells are organized in increasing energy levels, and each shell can hold a limited number of electrons. The innermost shell, closest to the nucleus, can hold a maximum of two electrons. The second and third shells can hold up to 8 electrons each.
The arrangement and behavior of the electrons can be explained using quantum mechanics and the concept of electron configurations. Electrons occupy the lowest energy levels available before filling higher levels, following specific rules and principles.
Rutherford’s model of the atom laid the foundation for our understanding of atomic structure. It demonstrated that the majority of the atom is empty space, with most of the mass concentrated in a small, dense nucleus. This model also explained the stability of the atom, as the positively charged protons in the nucleus are balanced by the negatively charged electrons.
However, Rutherford’s model did not consider certain aspects, such as the wave-like nature of electrons and the existence of subatomic particles like neutrinos. Subsequent advancements in the field of atomic structure, such as quantum theory and the discovery of additional subatomic particles, have refined our understanding of the atom further.
Key Experiments and Discoveries
One of the key experiments that led to the development of Rutherford’s nuclear model of the atom is the famous gold foil experiment, also known as the Rutherford scattering experiment. This experiment was conducted by Ernest Rutherford and his colleagues Hans Geiger and Ernest Marsden in 1909.
In this experiment, a beam of alpha particles (positively charged particles) was directed at a thin sheet of gold foil. According to the prevailing model at the time, the Thompson’s plum pudding model, an atom was thought to be a uniform, positively charged sphere with electrons embedded throughout its mass, much like plums in a pudding.
However, to their surprise, Rutherford and his team observed that most of the alpha particles passed straight through the gold foil, indicating that the atom was mostly empty space. This was expected since the alpha particles are much smaller than the size of the atom.
However, a small fraction of the alpha particles were deflected at large angles, and some even bounced directly back towards the source of the alpha particles. This unexpected result led Rutherford to propose a new model of the atom, now known as the nuclear model or the Rutherford model.
According to Rutherford’s model, the atom is mostly empty space, with a tiny, dense, and positively charged nucleus at its center. The electrons revolve around this nucleus in well-defined orbits. The deflections and bounces of the alpha particles were explained by the fact that they were interacting with the positive charge of the nucleus, which was much denser than previously believed.
This experiment and its results were crucial in shaping our understanding of the structure of the atom. It paved the way for further discoveries about the nucleus, such as the existence of protons and neutrons, as well as the development of the modern atomic theory.
Impact and Significance
Rutherford’s nuclear model had a profound impact on our understanding of the structure of the atom and laid the foundation for modern atomic theory. The model was proposed by New Zealand physicist Ernest Rutherford in 1911 based on his famous gold foil experiment.
One of the major impacts of Rutherford’s nuclear model was the realization that the atom is composed of a small, dense, and positively charged nucleus at the center. This was a radical departure from the prevailing model at the time, known as the Thomson model, which proposed that the positive charge was spread uniformly throughout the atom. Rutherford’s model demonstrated that the majority of the atom’s mass and positive charge is concentrated in a tiny volume, while most of the atom is empty space.
This discovery had significant implications for our understanding of the behavior of atoms and the interactions between particles. It explained why positive alpha particles, fired at a gold foil as part of Rutherford’s experiment, were deflected at large angles and even occasionally bounced back. This observation could only be explained if most of the atom’s mass was concentrated in a small, positively charged nucleus.
Another important consequence of Rutherford’s nuclear model was the development of the concept of atomic number. This model allowed scientists to explain the different chemical properties of elements based on the number of protons in their nuclei. This led to the understanding that elements are defined by their atomic number, which is the number of protons in the nucleus. Rutherford’s work laid the groundwork for the periodic table and the classification of elements based on their atomic numbers.
Overall, Rutherford’s nuclear model revolutionized our understanding of the atom, revealing its structure and leading to new insights into the behavior of matter. It paved the way for further discoveries in nuclear physics and particle interactions, and it continues to influence our understanding of atomic and subatomic particles to this day.
Topics related to Rutherfordʼs Nuclear Model
Rutherford Nuclear Model Wasn't Inspired by the Gold Foil Experiment – YouTube
Rutherford Nuclear Model Wasn't Inspired by the Gold Foil Experiment – YouTube
IGCSE Physics [Syllabus 5.1 – 5.2] Atomic physics – YouTube
IGCSE Physics [Syllabus 5.1 – 5.2] Atomic physics – YouTube
The Nuclear Atomic Model and Ernest Rutherford – YouTube
The Nuclear Atomic Model and Ernest Rutherford – YouTube
The Nuclear Model of the Atom | Physical Science – YouTube
The Nuclear Model of the Atom | Physical Science – YouTube
Rutherford's nuclear Model Of Atom – YouTube
Rutherford's nuclear Model Of Atom – YouTube
How Does The Nucleus Hold Together? – YouTube
How Does The Nucleus Hold Together? – YouTube
a nuclear physics primer – YouTube
a nuclear physics primer – YouTube
Rutherford's Model of An Atom – YouTube
Rutherford's Model of An Atom – YouTube
পরমাণুতে ইলেক্টনের কক্ষপথ How electrons move around the nucleus in bangla with animation Ep 48 – YouTube
পরমাণুতে ইলেক্টনের কক্ষপথ How electrons move around the nucleus in bangla with animation Ep 48 – YouTube
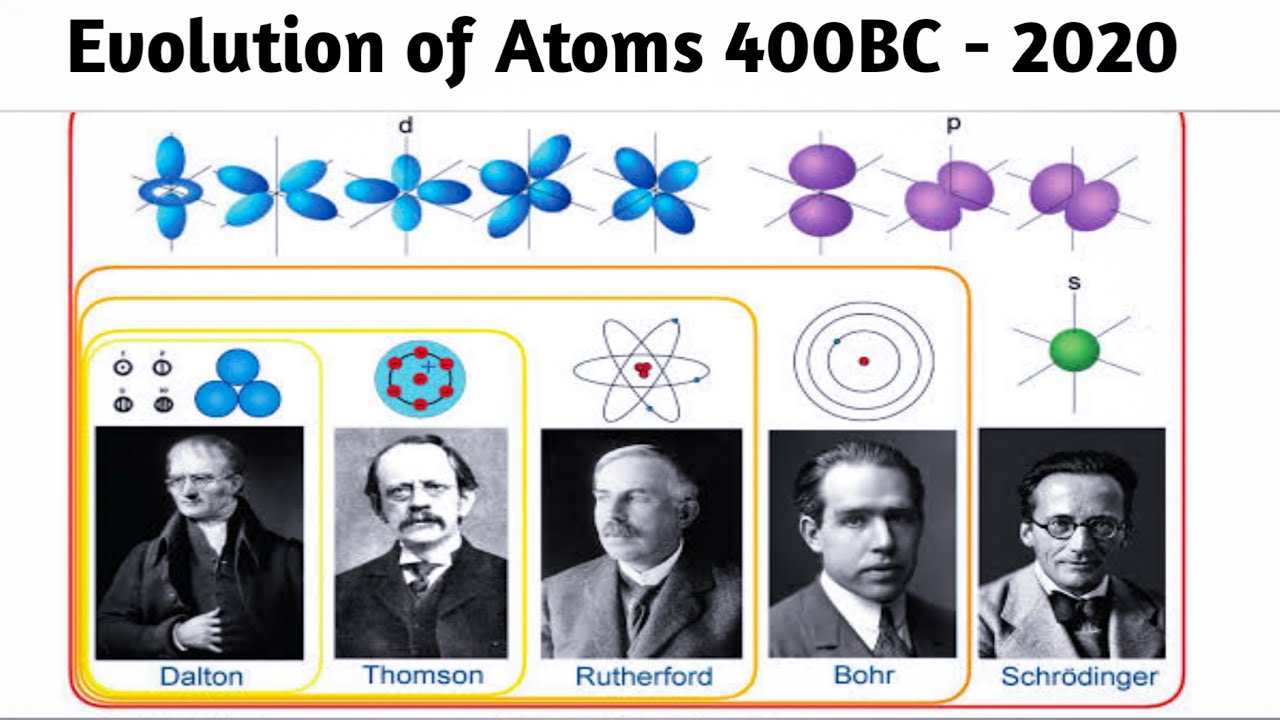
Evolution of Atomic Model 400 BC – 2020 | History of the atom Timeline, Atomic Theories – YouTube
Evolution of Atomic Model 400 BC – 2020 | History of the atom Timeline, Atomic Theories – YouTube

Konstantin Sergeevich Novoselov is a Russian-British physicist born on August 23, 1974. Novoselov is best known for his groundbreaking work in the field of condensed matter physics and, in particular, for his co-discovery of graphene. Novoselov awarded the Nobel Prize in Physics. Konstantin Novoselov has continued his research in physics and materials science, contributing to the exploration of graphene’s properties and potential applications.