Introduction to Boyle-Mariotte Law
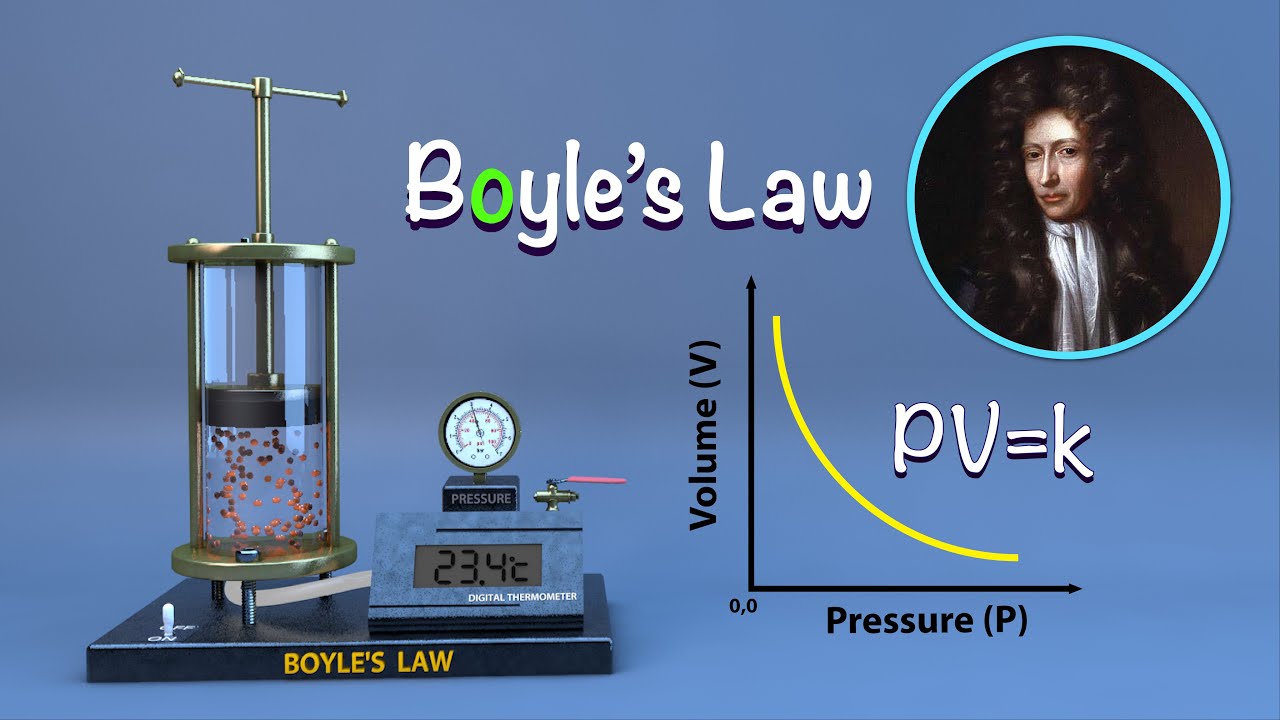
The Boyle-Mariotte law, also known as the Boyle’s law or the Mariotte’s law, is a fundamental principle in physics that describes the relationship between the pressure and volume of a gas, at a constant temperature. It was discovered independently by Robert Boyle in 1662 and later by Edme Mariotte in 1676.
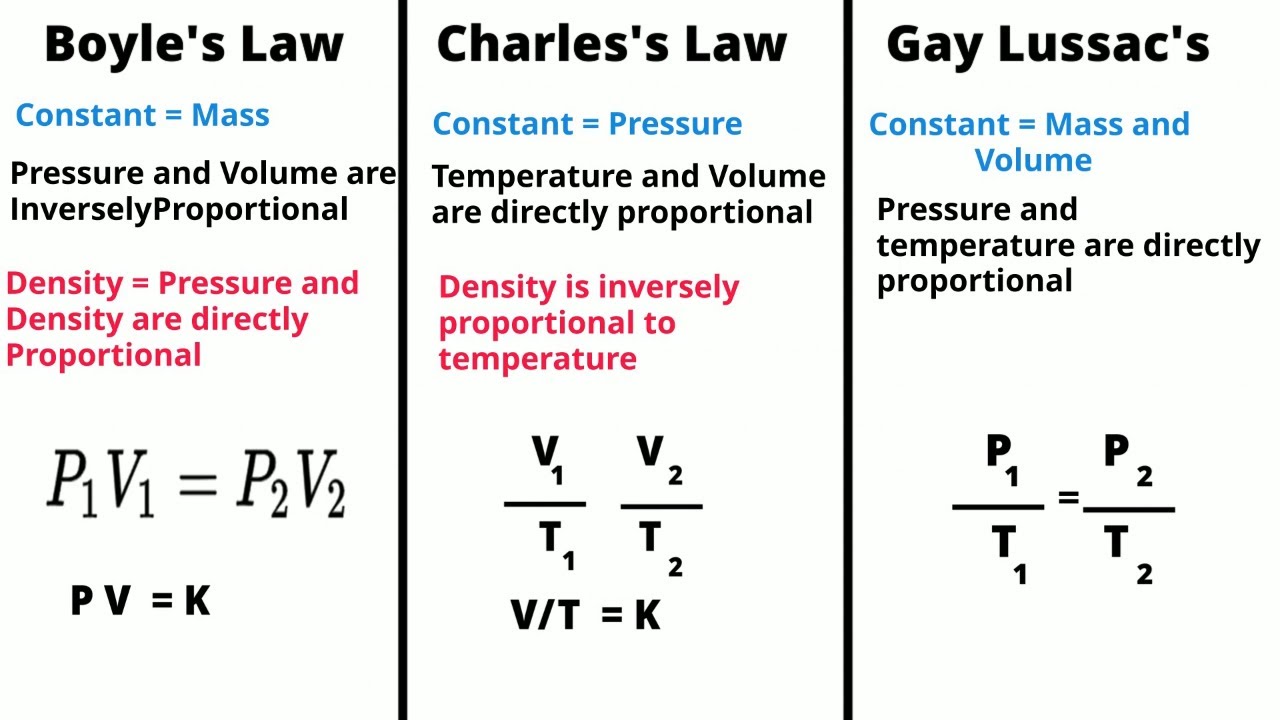
According to the Boyle-Mariotte law, the pressure of a gas is inversely proportional to its volume, while the temperature remains constant. In simpler terms, if the volume of a gas decreases, its pressure will increase, and vice versa, as long as the temperature remains constant.
Mathematically, Boyle-Mariotte law can be expressed as P₁V₁ = P₂V₂, where P₁ and V₁ represent the initial pressure and volume of the gas, and P₂ and V₂ represent the final pressure and volume of the gas.
This law holds true for an ideal gas, which is a gas that follows the assumptions of the kinetic theory of gases, such as having particles with no volume and undergoing elastic collisions.
The Boyle-Mariotte law has numerous applications in various fields of science and technology. It is particularly applicable in the study of gas behavior, such as in the design of gas containers, scuba diving, and even in the field of medicine, where it is used to understand the behavior of gases in the human body.
Overall, the Boyle-Mariotte law is a fundamental concept in understanding the properties and behavior of gases, providing a mathematical relationship between pressure and volume at a constant temperature.
Explanation of Boyle-Mariotte Law
The Boyle-Mariotte Law, also known as Boyle’s Law, states that at a constant temperature, the pressure of an ideal gas is inversely proportional to its volume. It describes the relationship between the pressure and volume of a gas sample when the temperature remains constant.
Mathematically, the Boyle-Mariotte Law can be expressed as P₁V₁ = P₂V₂, where P₁ and V₁ are the initial pressure and volume of the gas, and P₂ and V₂ are the final pressure and volume of the gas, respectively.
According to this law, when the volume of a gas decreases, the pressure increases proportionally. Similarly, when the volume of a gas increases, the pressure decreases proportionally. This inverse relationship helps explain various phenomena, such as why a balloon expands when filled with air and contracts when deflated.
Boyle’s Law is often used to predict the behavior of gases in different conditions. It is applicable to gases that behave ideally, meaning they follow the assumptions of the kinetic molecular theory. In practice, some gases, especially at high pressures or low temperatures, may deviate from this ideal behavior.
Application of Boyle-Mariotte Law
The Boyle-Mariotte Law, also known as Boyle’s Law, describes the relationship between the pressure and volume of a gas at constant temperature. It states that the volume of a given amount of gas is inversely proportional to its pressure, when temperature is held constant.
One practical application of the Boyle-Mariotte Law is in scuba diving. When a scuba diver descends into the water, the pressure increases due to the weight of the water above them. According to Boyle’s Law, this increase in pressure will cause a decrease in the volume of the gas in their scuba tank. As a result, the diver needs to adjust the regulator on their equipment to maintain a steady airflow of breathing gas as they go deeper, ensuring that they can still inhale the necessary amount of oxygen.
Another application can be seen in medical devices such as respiratory ventilators. These devices help patients breathe by delivering a controlled volume of gas. By utilizing the Boyle-Mariotte Law, the pressure in the ventilator can be adjusted to regulate the volume of gas delivered to the patient’s lungs. When the pressure is increased, the volume of gas decreases, and when the pressure is decreased, the volume increases.
Additionally, the Boyle-Mariotte Law is also applicable in the field of chemistry. For example, it is used in gas chromatography, a technique used to separate and analyze mixtures of gases. The law helps to understand how changes in pressure affect the volume of gases within the chromatography system, aiding in the accurate determination of gas concentrations in a sample.
In summary, the Boyle-Mariotte Law finds application in various fields such as scuba diving, medical devices, and chemistry, where the relationship between the pressure and volume of gases is crucial for practical purposes.
Limitations of Boyle-Mariotte Law
The Boyle-Mariotte Law, also known as the Boyle’s Law, states that the pressure and volume of a gas are inversely proportional at a constant temperature. While this law is useful in understanding the behavior of gases, it has some limitations which include:
1. Temperature Dependency: The Boyle-Mariotte Law assumes that the temperature remains constant during the process. However, in real-life scenarios, temperature changes can significantly impact the behavior of gases, leading to deviations from the ideal behavior predicted by the law.
2. Limited Applicability: The Boyle-Mariotte Law is only applicable to ideal gases, which follow certain assumptions such as negligible intermolecular forces and infinite molecular volume. Real gases, on the other hand, do not always obey this law due to factors like molecular interactions and finite molecular volume.
3. High Pressure and Low Temperature Deviations: The law becomes less accurate at high pressures and low temperatures. At extreme conditions, gases can deviate from the ideal behavior and exhibit non-ideal characteristics, such as condensing into a liquid or even solidifying.
4. Time Dependency: The law assumes that the change in volume occurs instantaneously when the pressure changes. In reality, gases take some time to adjust to changes in pressure, and therefore, the law may not accurately predict the behavior of gases under such dynamic conditions.
5. Lack of Volume Limitation: The Boyle-Mariotte Law does not account for situations where the volume of a gas approaches zero or becomes extremely large. At these limits, the law fails to provide accurate predictions.
6. Isothermal Assumption: The law assumes that the process occurs at a constant temperature, also known as an isothermal process. However, many real-life processes involve temperature changes, and the law’s applicability is limited in such cases.
7. Non-Gaseous Systems: The Boyle-Mariotte Law is strictly applicable to gases and may not be valid for other states of matter, such as liquids or solids. The law fails to account for the unique properties and behaviors of non-gaseous substances.
While the Boyle-Mariotte Law has its limitations, it still serves as an important concept in understanding the behavior of gases under certain conditions.
Importance of Boyle-Mariotte Law in Physics
The Boyle-Mariotte law, named after the scientists Robert Boyle and Edme Mariotte, is an important law in the field of physics. It describes the relationship between the pressure and volume of a gas at constant temperature. According to the Boyle-Mariotte law, the pressure of a gas is inversely proportional to its volume when the temperature remains constant.
The law can be mathematically represented as follows:
P1V1 = P2V2
where P1 and V1 represent the initial pressure and volume of the gas, and P2 and V2 represent the final pressure and volume of the gas.
The importance of the Boyle-Mariotte law lies in its applications in various areas of physics, particularly in the study of gases. It helps to explain phenomena such as the behavior of gases under different pressures and volumes.
One significant application of the law is in the field of thermodynamics. It provides a fundamental understanding of how gases behave when subjected to changes in pressure and volume. This knowledge is crucial for various practical applications, including the design of engines, refrigeration systems, and various industrial processes.
The Boyle-Mariotte law is also important in the study of gas laws and the ideal gas equation. It serves as a foundation for understanding other laws, such as Charles’ law and Gay-Lussac’s law, which describe the relationship between temperature and volume, and temperature and pressure, respectively.
Overall, the Boyle-Mariotte law is an essential concept in physics, providing insights into gas behavior and facilitating the understanding and prediction of various phenomena related to gases. It plays a significant role in various scientific and technological applications, making it a fundamental principle in the field of physics.
Topics related to Boyle–Mariotte Law
BOYLE'S LAW | Animation – YouTube
BOYLE'S LAW | Animation – YouTube
Boyle's Law Practice Problems – YouTube
Boyle's Law Practice Problems – YouTube
Boyle's Law Demonstrations – YouTube
Boyle's Law Demonstrations – YouTube
The law of Boyle and Mariotte – experiment – YouTube
The law of Boyle and Mariotte – experiment – YouTube
Boyle's Law – Physics A-level Required Practical – YouTube
Boyle's Law – Physics A-level Required Practical – YouTube
Gas Laws-Boyle's-Charles's-Gay Lussac's – YouTube
Gas Laws-Boyle's-Charles's-Gay Lussac's – YouTube
Boyle's Law Experiment – Balloon Test – Science Projects for Kids | Educational Videos by Mocomi – YouTube
Boyle's Law Experiment – Balloon Test – Science Projects for Kids | Educational Videos by Mocomi – YouTube
Boyle's law: Explanation, Limitations and Applications – Explained Details (Animation) – YouTube
Boyle's law: Explanation, Limitations and Applications – Explained Details (Animation) – YouTube
Boyle's Law Example Problems – YouTube
Boyle's Law Example Problems – YouTube
CHARLES' LAW | Animation – YouTube
CHARLES' LAW | Animation – YouTube

Konstantin Sergeevich Novoselov is a Russian-British physicist born on August 23, 1974. Novoselov is best known for his groundbreaking work in the field of condensed matter physics and, in particular, for his co-discovery of graphene. Novoselov awarded the Nobel Prize in Physics. Konstantin Novoselov has continued his research in physics and materials science, contributing to the exploration of graphene’s properties and potential applications.