Introduction to Nuclear Magnetic Resonance (NMR)
Nuclear Magnetic Resonance (NMR) is a powerful analytical technique used in chemistry, physics, and medicine to study the structure, dynamics, and composition of molecules. It is based on the principles of nuclear spin and magnetic fields.
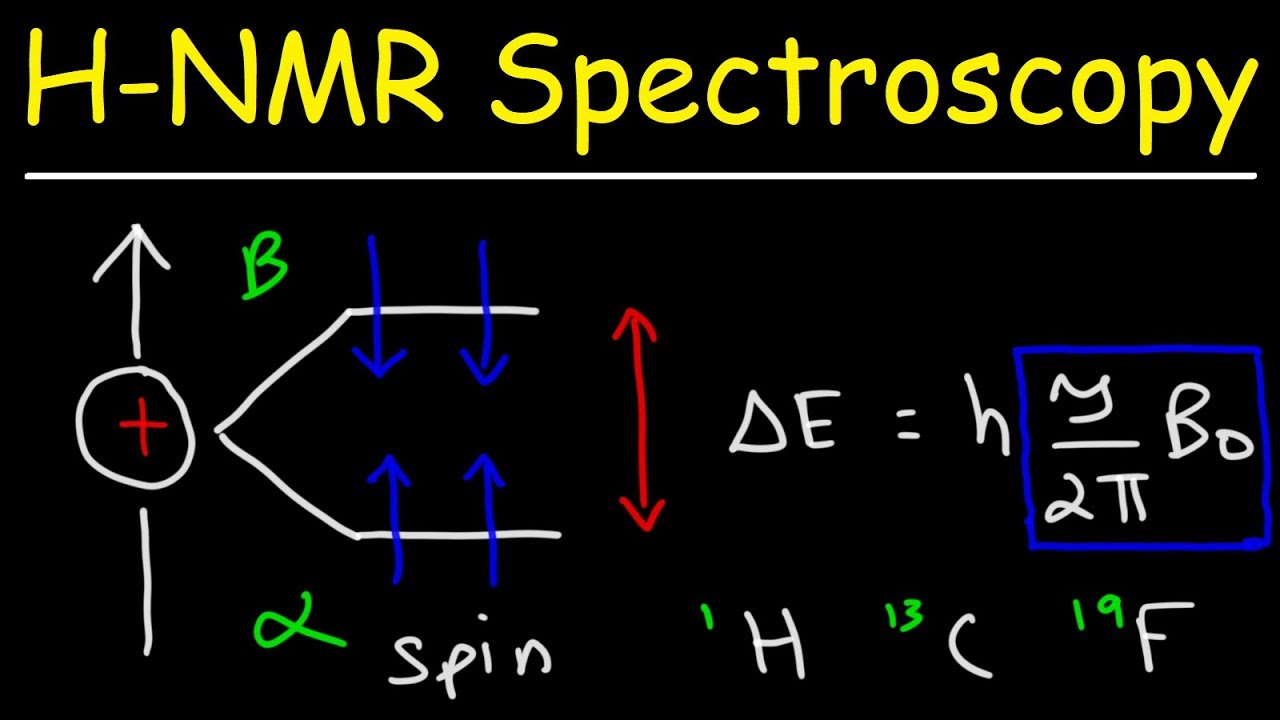
NMR works by exploiting the magnetic properties of certain atomic nuclei, typically those of atoms such as hydrogen (1H), carbon-13 (13C), or phosphorus-31 (31P). These nuclei possess a property called “spin,” which generates a tiny magnetic field. In the absence of an external field, these nuclear spins are randomly oriented. However, when placed in a strong external magnetic field, the spins align either parallel or anti-parallel to the field.
When a magnetic field is applied, the nuclear spins absorb and emit energy at specific frequencies characteristic of the atom and its chemical environment. By subjecting the sample to a range of frequencies and measuring the absorbed energy, NMR can provide information about the molecular structure, such as bond connectivity, stereochemistry, and molecular dynamics.
NMR spectroscopy is usually performed by placing the sample in a powerful magnet and irradiating it with radiofrequency (RF) pulses. By measuring the response of the sample to these pulses, information about the molecular properties can be obtained. The most common type of NMR experiment is proton NMR (1H NMR), which uses the hydrogen nuclei in molecules to obtain structural information. Other types include carbon-13 NMR (13C NMR) and other nuclei-specific experiments.
NMR has a wide range of applications in various fields. In chemistry, it is used for chemical structure determination, studying reaction kinetics, and observing molecular interactions. In biology and medicine, NMR is employed for biomolecule structure determination, drug development, and metabolic studies. NMR also finds applications in materials science, physics, and geology.
Overall, NMR is a versatile and non-destructive technique that provides valuable insights into the properties of molecules, making it an indispensable tool for scientists in many fields.
Principles of NMR in Physics
Nuclear Magnetic Resonance (NMR) is a powerful technique used in physics to study the properties of atomic nuclei in a magnetic field. It is based on the principle of the interaction between nuclear spins and a magnetic field.
Here are some important principles of NMR in physics:
1. Nuclear spins: Nuclei of certain atoms, such as hydrogen (1H), carbon-13 (13C), and nitrogen-15 (15N), possess an intrinsic property known as nuclear spin. This spin gives rise to a tiny magnetic moment, which interacts with an external magnetic field.
2. Energy levels: In the absence of a magnetic field, nuclear spins can exist in different energy levels. When a magnetic field is applied, these energy levels split into multiple states, known as Zeeman splitting. The energy difference between these states is directly proportional to the strength of the magnetic field.
3. Resonance condition: By applying an additional radiofrequency (RF) electromagnetic field, nuclei can be excited from the lower to higher energy states. The resonance condition occurs when the frequency of the RF field matches the energy difference between the nuclear spin states. This causes a transition between the energy levels.
4. Absorption and emission: When the resonance condition is satisfied, nuclei absorb energy from the RF field and jump to higher energy states. Subsequently, they relax back to the lower energy states by emitting electromagnetic radiation. The frequency of this emitted radiation is known as the resonance frequency.
5. Chemical shift: In addition to the external magnetic field, the local chemical environment of a nucleus also affects its resonance frequency. This phenomenon is called chemical shift and provides information about the molecular structure and bonding of the atom being studied.
6. NMR spectroscopy: NMR spectroscopy is a widely used technique in which a sample is exposed to a magnetic field and subjected to a range of RF frequencies. By measuring the absorption or emission of radiation at different frequencies, a detailed spectrum can be obtained. This spectrum provides information about the abundance, chemical bonding, and molecular structure of the nuclei in the sample.
7. Magnetic resonance imaging (MRI): MRI is a medical imaging technique that utilizes NMR principles. It uses a strong magnetic field and RF pulses to obtain detailed images of the internal structures of the human body. By manipulating the RF pulses and magnetic field gradients, different types of tissue can be selectively imaged, making MRI a versatile diagnostic tool.
Overall, NMR in physics is a fundamental tool for understanding the behavior of atomic nuclei in a magnetic field. It has a wide range of applications in various fields, including chemistry, biology, material science, and medical diagnostics.
Applications of NMR in Physics
Nuclear Magnetic Resonance (NMR) is a powerful spectroscopic technique that has found a wide range of applications in various fields of physics. Some of the key applications of NMR in physics are:
1. Magnetic Resonance Imaging (MRI): NMR is extensively used in the field of medical physics for non-invasive imaging of soft tissues in the human body. MRI uses the principles of NMR to create detailed images of the internal structures of the body.
2. Materials Science: NMR spectroscopy is used to study the physical properties and behavior of materials at the atomic and molecular level. It provides information about the structure, dynamics, and interactions of materials, helping in the design and characterization of new materials.
3. Solid-State Physics: NMR is widely used in the study of solid-state physics, providing valuable insights into various properties of solids such as crystal structures, electronic band structures, and magnetic ordering.
4. Quantum Computing: NMR techniques have been employed in quantum computing research, particularly in the field of nuclear spin quantum computing. NMR spectroscopy is used to manipulate and control the quantum states of nuclear spins in molecules, which can be used to perform quantum computations.
5. Fundamental Research: NMR has been used in fundamental research in various areas of physics such as quantum mechanics, condensed matter physics, and high-energy physics. It provides a powerful tool to probe the intrinsic properties of atomic and molecular systems, allowing for the study of fundamental physical phenomena.
6. Superconductivity: NMR techniques are used to investigate the behavior of superconducting materials, providing insights into the mechanism of superconductivity and the properties of superconductors at different temperatures and magnetic fields.
7. Magnetic Resonance Spectroscopy: NMR spectroscopy is widely used to study the chemical composition and structure of molecules. It is employed in various fields such as organic chemistry, biochemistry, and environmental science to analyze and identify compounds, study molecular dynamics, and investigate the interactions between molecules.
Overall, NMR has proven to be a versatile and powerful tool in physics, offering valuable insights into the structure, dynamics, and properties of various systems at the atomic and molecular level.
Advantages and Limitations of NMR
Advantages of NMR:
1. Non-destructive technique: NMR does not require sample preparation or labeling, as it detects the inherent magnetic properties of atomic nuclei. This allows for the analysis of samples without altering their chemical composition.
2. High sensitivity: NMR can detect very small quantities of substances, making it a valuable tool for analyzing low-concentration samples.
3. Versatility: NMR can be used to analyze a wide range of materials, including liquids, solids, and gases. It is applicable to various fields such as chemistry, biochemistry, medicine, and materials science.
4. Structural analysis: NMR provides information about the molecular structure, conformation, and dynamics of compounds, allowing researchers to determine the connectivity of atoms and study molecular interactions.
5. Quantitative analysis: NMR can be used to quantify the amount of a specific compound in a mixture by measuring the intensity of the NMR signal. This makes it useful for determining the concentration of substances in a sample.
Limitations of NMR:
1. Expensive equipment and maintenance: NMR spectrometers are expensive to purchase and maintain. They require skilled operators and regular servicing to ensure accurate and reliable results.
2. Time-consuming: NMR experiments can take a long time to acquire data, especially for complex samples. This can limit its applicability in time-sensitive research or high-throughput analysis.
3. Complexity of data interpretation: Analyzing NMR spectra often requires expertise in spectral interpretation and complex mathematical techniques. The presence of multiple peaks, overlapping signals, and complex spectral patterns can make data analysis challenging.
4. Limited sensitivity for certain elements: While NMR is highly sensitive for hydrogen and carbon nuclei, it may have lower sensitivity for other elements, such as oxygen, nitrogen, and metals. This can limit its usability in certain applications.
5. Sample limitations: NMR requires samples to be in a liquid or solid state and may be less applicable to samples that are sensitive to the strong magnetic fields used in the technique. Additionally, large sample volumes may be required for accurate analysis, which may pose challenges for limited sample availability.
Conclusion and Future Developments in NMR
Nuclear magnetic resonance (NMR) has significantly advanced our understanding of the structure and dynamics of molecules and materials. It has become a powerful tool in numerous fields such as chemistry, biochemistry, materials science, and medicine.
In conclusion, NMR has revolutionized the study of molecular interactions, providing detailed information about atomic-level structures, chemical bonding, and molecular dynamics. It has helped elucidate the structures of complex molecules, diagnose diseases, and determine the properties of materials.
Moving forward, there are several potential future developments in NMR that hold great promise. One area of future development is in the improvement of NMR instrumentation and techniques. Advances in hardware, such as higher magnetic field strengths and more sensitive detectors, will allow for higher resolution and more detailed analysis of molecules and materials. Improved pulse sequences and data acquisition methods will also enhance the capabilities of NMR.
Another area of future development is the expansion of NMR applications. NMR is already widely used in fields such as chemistry and biochemistry, but there is the potential for it to be utilized in new areas. For example, NMR could play a vital role in the study of nanomaterials, catalysis, and drug discovery. Additionally, NMR could be further integrated with other spectroscopic and imaging techniques to provide a more comprehensive understanding of complex systems.
Lastly, the development of new and advanced computational methods for NMR data analysis will be crucial for the future of the field. Machine learning and artificial intelligence techniques can be employed to extract valuable information from complex NMR datasets, enabling quicker and more accurate analysis.
In conclusion, NMR has already made remarkable contributions to various scientific fields, and its future developments hold great promise. Continued advancements in technology, expanded applications, and improved data analysis methods will undoubtedly lead to further breakthroughs and discoveries using NMR.
Topics related to Nuclear magnetic resonance (NMR)
NMR spectroscopy visualized – YouTube
NMR spectroscopy visualized – YouTube
What is Nuclear Magnetic Resonance (NMR)? // HSC Chemistry – YouTube
What is Nuclear Magnetic Resonance (NMR)? // HSC Chemistry – YouTube
NMR Spectroscopy – YouTube
NMR Spectroscopy – YouTube
Using Nuclear Magnetic Resonance (NMR) spectroscopy to identify electrochemical reactions products – YouTube
Using Nuclear Magnetic Resonance (NMR) spectroscopy to identify electrochemical reactions products – YouTube
Nuclear Magnetic Resonance – YouTube
Nuclear Magnetic Resonance – YouTube
Using Nuclear Magnetic Resonance (NMR) spectroscopy to characterise ball bearing molecules – YouTube
Using Nuclear Magnetic Resonance (NMR) spectroscopy to characterise ball bearing molecules – YouTube
Nuclear Magnetic Resonance: Principles and Applications of NMR – YouTube
Nuclear Magnetic Resonance: Principles and Applications of NMR – YouTube
Basic Introduction to NMR Spectroscopy – YouTube
Basic Introduction to NMR Spectroscopy – YouTube
NMR Spectroscopy: The Ultimate Guide for Beginners || Nuclear Magnetic Resonance|| NMR – YouTube
NMR Spectroscopy: The Ultimate Guide for Beginners || Nuclear Magnetic Resonance|| NMR – YouTube
Nuclear Magnetic Resonance (NMR) – YouTube
Nuclear Magnetic Resonance (NMR) – YouTube

Konstantin Sergeevich Novoselov is a Russian-British physicist born on August 23, 1974. Novoselov is best known for his groundbreaking work in the field of condensed matter physics and, in particular, for his co-discovery of graphene. Novoselov awarded the Nobel Prize in Physics. Konstantin Novoselov has continued his research in physics and materials science, contributing to the exploration of graphene’s properties and potential applications.