Introduction to Ammonium Nitrate
Ammonium nitrate is a chemical compound with the formula NH4NO3. It is commonly used in agriculture as a high-nitrogen fertilizer. Ammonium nitrate is highly soluble in water and provides plants with essential nitrogen for growth.
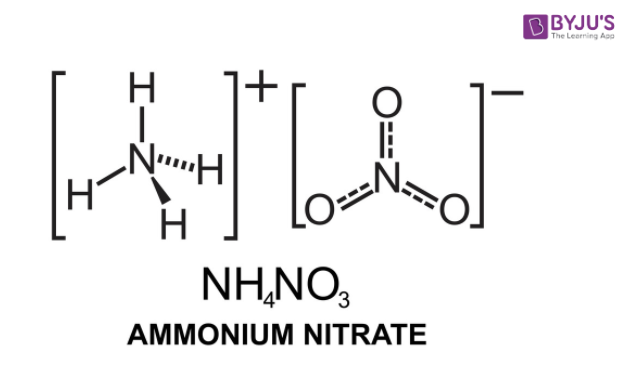
In terms of its chemical structure, ammonium nitrate consists of one nitrogen atom (N) bonded to three hydrogen atoms (H) in the ammonium ion (NH4+), and one nitrate ion (NO3-) composed of one nitrogen atom and three oxygen atoms. The compound itself is a white crystalline solid and has a melting point of around 170 degrees Celsius.
One of the important properties of ammonium nitrate is its ability to undergo a process called endothermic decomposition when heated. This means that it absorbs heat from its surroundings as it decomposes into various gases, including nitrogen oxide (NO), water vapor (H2O), and oxygen gas (O2). This decomposition process is highly exothermic and can lead to explosive reactions under certain conditions.
Due to its explosive potential, ammonium nitrate has also found applications outside of agriculture. It is commonly utilized in the production of explosives, such as in mining and construction industries. However, its use as an explosive is tightly regulated in many countries due to safety concerns.
In summary, ammonium nitrate is a versatile chemical compound that serves as a fertilizer in agriculture and has applications in the manufacturing of explosives. Understanding its properties and potential hazards is crucial for its safe handling and usage.
Chemical Structure of Ammonium Nitrate
The chemical structure of ammonium nitrate (NH4NO3) consists of two components: the ammonium ion (NH4+) and the nitrate ion (NO3-).
The ammonium ion consists of a central nitrogen atom bonded to four hydrogen atoms. The nitrogen atom forms three single covalent bonds with three of the hydrogen atoms, while the fourth hydrogen atom is connected via a coordinate covalent bond, meaning the electron pair is donated by the nitrogen atom to form the bond.
The nitrate ion consists of a central nitrogen atom bonded to three oxygen atoms. The nitrogen atom forms a single covalent bond with one oxygen atom and a double covalent bond with another oxygen atom. The remaining oxygen atom is connected via a coordinate covalent bond with the nitrogen atom.
In the overall structure of ammonium nitrate, the ammonium ion and the nitrate ion are held together by ionic bonds. The nitrogen atom in the ammonium ion forms a coordinate covalent bond with one of the oxygen atoms in the nitrate ion, creating a stable ionic compound.
Therefore, the chemical formula of ammonium nitrate, NH4NO3, represents the arrangement of one ammonium ion and one nitrate ion in the structure.
Properties and Uses of Ammonium Nitrate
Properties:
1. Chemical formula: NH4NO3
2. Molecular weight: 80.043 g/mol
3. Appearance: White crystalline solid
4. Density: 1.725 g/cm3
5. Melting point: 169.6 °C
6. Boiling point: Decomposes above 210 °C
7. Solubility: Highly soluble in water
8. pH: Neutral (7) in aqueous solution
Uses:
1. Fertilizer: Ammonium nitrate is a widely used nitrogen-containing fertilizer due to its high nitrogen content. It provides plants with essential nutrients for growth and development. It can be applied directly or mixed with other fertilizers to enhance the nutrient content of the soil.
2. Explosives: Ammonium nitrate is a key ingredient in the production of industrial explosives such as dynamite and ANFO (ammonium nitrate/fuel oil). These explosives are widely used in mining, construction, and demolition industries. Ammonium nitrate provides the necessary oxygen for rapid combustion when mixed with a suitable fuel, such as diesel oil.
3. Cold packs: Ammonium nitrate is used in some cold packs or instant ice packs. These packs contain separate compartments that, when broken, mix ammonium nitrate with water. The dissolution of ammonium nitrate in water is an endothermic process that absorbs heat from the surroundings, rapidly cooling the pack and creating a cold sensation.
4. Food preservation: Ammonium nitrate is sometimes used as a food preservative. It is added to certain cheeses, processed meats, and other food products to inhibit bacterial growth and extend their shelf life.
5. Pyrotechnics: Ammonium nitrate can be used in fireworks and pyrotechnics to provide an oxygen source for combustion reactions. It is commonly combined with various fuels and metal salts to produce vibrant colors and visual effects.
6. Laboratory reagent: Ammonium nitrate can be used as a reagent or analytical standard in various chemistry laboratories. It is used in specific reactions or experiments that require a source of ammonium ions or nitrate ions.
It is important to note that while ammonium nitrate has many useful applications, it must be handled with care due to its potential explosive properties.
Safety Concerns and Hazards associated with Ammonium Nitrate
Ammonium nitrate, a chemical compound commonly used in fertilizers, can pose a variety of safety concerns and hazards due to its physical and chemical properties. Some of the major safety concerns associated with ammonium nitrate include:
1. Explosive Properties: Under certain conditions, ammonium nitrate can become highly explosive. It is classified as an explosive substance and can detonate if exposed to high temperatures, shock, or other sources of ignition. Accidental explosions can lead to significant damage and pose a threat to human life and property.
2. Oxidizer: Ammonium nitrate is a potent oxidizing agent, which means it supports combustion and can increase the intensity of fires. When involved in a fire, it can cause rapid spread and intensification of flames, making firefighting efforts challenging and increasing the risk to personnel.
3. Compatibility with Other Substances: Ammonium nitrate can react dangerously with various fuels, organic materials, and other chemicals. Mixing it with flammable or combustible substances can result in highly reactive and potentially explosive mixtures. Special care is needed when storing, handling, or transporting ammonium nitrate in proximity to other materials.
4. Heat Generation: Ammonium nitrate is hygroscopic, meaning it absorbs moisture from the air. The absorption of moisture can cause the formation of clumps or lumps, leading to heat buildup. If large quantities of ammonium nitrate are stored together and become overheated, the risk of a thermal event, such as self-ignition or explosion, increases.
5. Toxic Gas Release: In certain situations, ammonium nitrate can decompose, releasing toxic gases, such as nitrogen oxides (NOx). Exposure to these gases can irritate the respiratory system, cause difficulty in breathing, and have other harmful health effects.
To mitigate these safety concerns and hazards, care must be taken when handling, storing, and transporting ammonium nitrate. Proper storage conditions, handling procedures, and adherence to safety guidelines and regulations are essential to prevent accidents and protect personnel and the surrounding environment.
Environmental Impact of Ammonium Nitrate
Ammonium nitrate is a widely-used chemical compound in chemistry, with significant environmental impacts. Here are some of the key environmental concerns associated with ammonium nitrate:
1. Water pollution: When ammonium nitrate is used as a fertilizer, excess amounts can leach into water bodies such as rivers, lakes, and groundwater. The high levels of nitrogen present in ammonium nitrate can cause eutrophication, where excessive nutrients promote the overgrowth of algae and aquatic plants. This can deplete oxygen levels in the water, harming fish and other aquatic organisms.
2. Air pollution: Ammonium nitrate can also contribute to air pollution. During its manufacturing process, emissions of nitrous oxides (NOx) are released, which are known to be a major source of air pollution contributing to the formation of smog and ground-level ozone. NOx emissions are also associated with respiratory problems and can contribute to the formation of acid rain.
3. Greenhouse gas emissions: The production and use of ammonium nitrate also contribute to greenhouse gas emissions. The manufacturing process often involves the burning of fossil fuels, which release carbon dioxide (CO2), a greenhouse gas that contributes to climate change.
4. Explosive properties: While not directly related to environmental impact, it is important to mention that ammonium nitrate has explosive properties. Improper storage or handling of ammonium nitrate can result in accidents, such as the explosion in the Beirut port in 2020. These incidents can cause significant damage to the environment, including air and water pollution, destruction of habitats, and loss of biodiversity.
It is worth noting that environmental impact can be mitigated through proper handling and disposal practices, as well as the implementation of technologies to reduce emissions during manufacturing processes. Additionally, transitioning to sustainable and environmentally-friendly alternatives can help reduce the environmental footprint associated with ammonium nitrate use.

Abigail Gutmann Doyle is a renowned Organic chemistry professor in Los Angeles. Her research focuses on the development of new chemical transformations in organic chemistry. She has won awards such as: Bayer Early Excellence in Science Award, Phi Lambda Upsilon National Fresenius Award, Presidential Early Career Award for Scientists and Engineers, BMS Unrestricted Grant in Synthetic Organic Chemistry.