Introduction
Chemistry is the branch of science that deals with the properties, composition, structure, and changes of matter. It is often referred to as the central science because it connects and bridges concepts from other scientific disciplines such as physics, biology, and geology. Chemistry is involved in every aspect of our daily lives, from the food we eat to the products we use, and even the reactions occurring in our bodies.
Chemists study the different types of matter, including elements, compounds, and mixtures, and how they interact with each other. They explore the behavior and transformations of substances at the molecular and atomic levels, deciphering the fundamental principles that govern the formation, stability, and reactivity of different chemical compounds.
Chemistry is divided into several branches, including organic chemistry, inorganic chemistry, physical chemistry, analytical chemistry, and biochemistry. Each branch focuses on specific areas of study, such as the composition and synthesis of organic compounds, the properties of inorganic substances, the thermodynamics and kinetics of chemical reactions, the analysis and identification of substances, and the study of chemical processes in living organisms.
Chemistry has wide-ranging applications in various fields, including medicine, materials science, pharmaceuticals, environmental science, and energy production. It plays a crucial role in developing new drugs, designing advanced materials, understanding and controlling pollution, and developing sustainable energy sources.
In summary, chemistry is a fundamental scientific discipline that explores the composition, properties, and changes of matter. It provides a solid foundation for understanding the natural world and has significant implications for technological advancements and improving our quality of life.
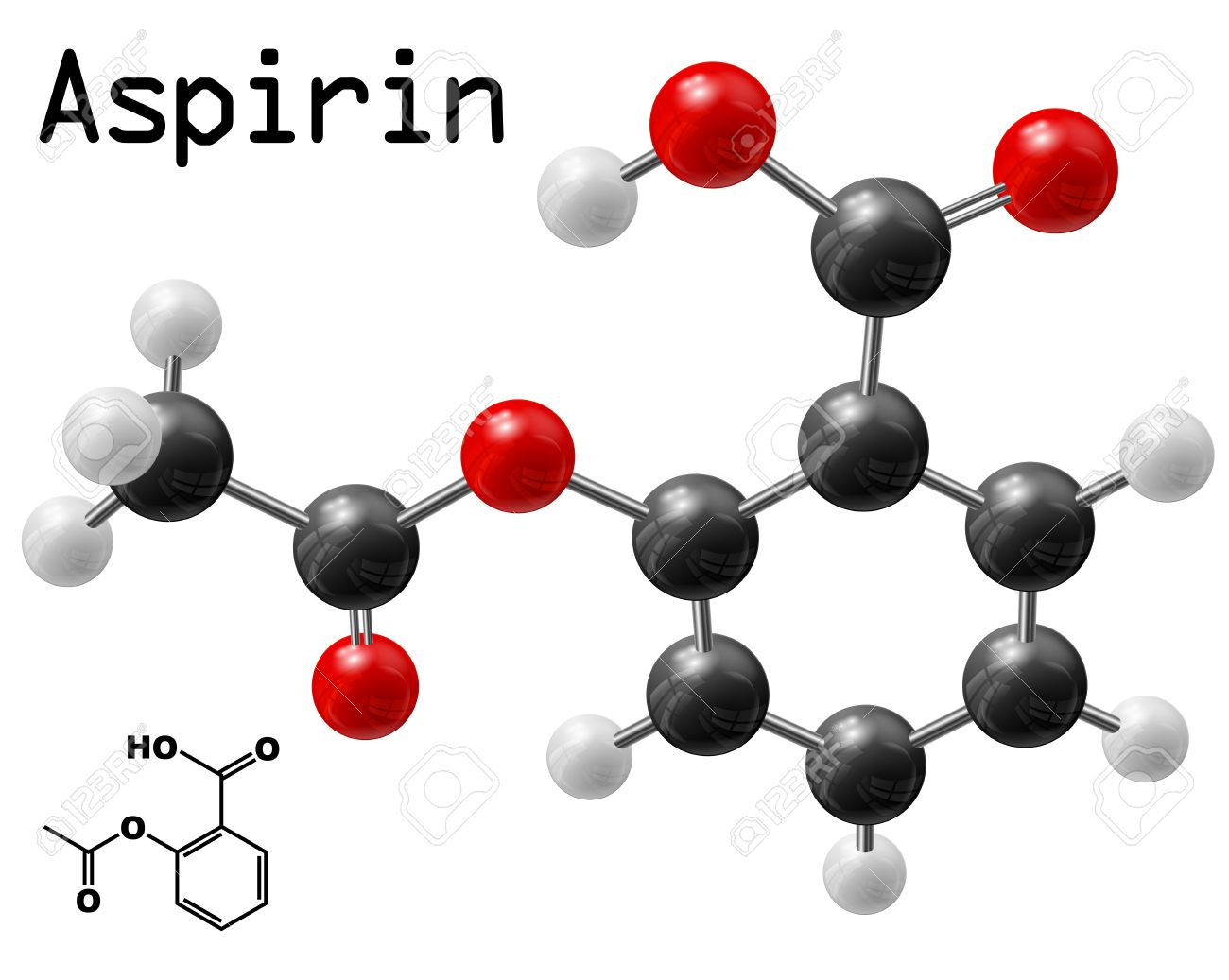
Chemical Structure of Aspirin
The chemical structure of aspirin, also known as acetylsalicylic acid, can be represented as follows:
O
||
HO–C–O–C–CH3
||
O
This is a condensed structural formula, which shows the connectivity of atoms but does not represent the spatial arrangement of atoms in three dimensions. In this structure, the red atoms represent oxygen, the black atoms represent carbon, the white atoms represent hydrogen, and the blue atom represents methyl (CH3) group.
Aspirin is derived from salicylic acid by acetylation, where an acetyl group (CH3CO-) replaces the hydrogen atom of the hydroxyl group (-OH) of salicylic acid. The presence of the acetyl group in aspirin provides certain properties, such as improved stability and reduced irritation to the stomach lining.
It is important to note that the structure shown here reflects the basic structure of aspirin, but there may be variations in the arrangement of atoms or different forms of the molecule depending on different factors, such as the crystalline form or the presence of counterions.
Properties of Aspirin
Aspirin, also known as acetylsalicylic acid, is a common medication that possesses several properties in chemistry. Some of these properties include:
1. Chemical formula: Aspirin has the chemical formula C9H8O4, indicating that it is composed of 9 carbon atoms, 8 hydrogen atoms, and 4 oxygen atoms.
2. Molecular weight: The molecular weight of aspirin is approximately 180.16 grams/mol.
3. Physical state: Aspirin is a white crystalline powder, solid at room temperature.
4. Melting point: Aspirin has a melting point of around 135-136 degrees Celsius.
5. Reactivity: Aspirin is a carboxylic acid derivative and is known to undergo several chemical reactions. It can be hydrolyzed by water, forming salicylic acid and acetic acid. It can also react with bases, such as sodium hydroxide, to form a salt and water.
6. Acidity: Aspirin is a weak acid with a pKa value of around 3.5, meaning that it partially dissociates in water to release hydrogen ions.
7. Solubility: Aspirin is sparingly soluble in water, but more soluble in organic solvents such as ethanol and acetone.
8. Stability: Aspirin is relatively stable under normal storage conditions. However, it can hydrolyze over time and degrade in the presence of moisture, heat, or exposure to light, converting into acetic acid and salicylic acid.
9. Pharmacological properties: Aspirin is a nonsteroidal anti-inflammatory drug (NSAID) that works by irreversibly inhibiting the enzyme cyclooxygenase, which is involved in the production of prostaglandins. It possesses analgesic, anti-inflammatory, and antipyretic properties.
10. Biological metabolism: In the body, aspirin is rapidly hydrolyzed into salicylic acid, which is further metabolized in the liver and excreted primarily in the urine.
It is important to note that the properties described above are based on general characteristics of aspirin. The specific values and behavior may vary depending on various factors such as purity, conditions, and concentration of the substance.
Uses and Applications of Aspirin
Aspirin, or acetylsalicylic acid, has various uses and applications in chemistry. Some of these include:
1. Analgesic and anti-inflammatory agent: Aspirin is commonly used as a pain reliever and anti-inflammatory medication due to its ability to inhibit prostaglandin synthesis, which reduces pain and inflammation. Its chemical structure allows it to inhibit the action of the enzyme cyclooxygenase, which is involved in the production of prostaglandins.
2. Platelet aggregation inhibitor: Aspirin has the ability to inhibit platelet aggregation, making it useful in preventing blood clot formation. It irreversibly acetylates cyclooxygenase-1 (COX-1) enzyme in platelets, inhibiting the synthesis of thromboxane A2, a platelet aggregator. This property is utilized in the prevention of heart attacks and strokes.
3. Buffer preparation: Aspirin can be used as a weak acid to prepare buffers in certain laboratory experiments. Its acidic properties allow it to stabilize the pH of a solution. This can be particularly useful in biochemical and biological assays where maintaining a specific pH range is critical.
4. Ester hydrolysis: Aspirin is an ester, and it can undergo hydrolysis in the presence of water or hydroxide ions. This property is utilized in various chemical reactions and synthesis processes. For example, aspirin can be hydrolyzed to produce salicylic acid, which is often used in the production of various chemicals and pharmaceuticals.
5. Standard for acid-base titrations: Aspirin can be used as a standard in acid-base titrations to determine the concentration of a base. Its reaction with a strong base, such as sodium hydroxide, can be followed by the color change of a pH indicator, allowing for titration calculations.
6. Analytical reagent: Aspirin can act as a reagent in certain analytical techniques. For example, it can be used in the spectrophotometric determination of iron ions, where iron(III) ions react with aspirin to form a colored complex that can be measured photometrically.
It is important to note that while aspirin has these applications and uses in chemistry, it is primarily known for its medicinal value as a pain reliever and anti-inflammatory drug.
Conclusion
In conclusion, chemistry is a fundamental science that explores the composition, structure, properties, and reactions of matter. It helps us understand the natural world around us, from the behavior of atoms and molecules to the formation of compounds and the dynamics of chemical reactions. Chemistry plays a crucial role in many aspects of our lives, including medicine, industry, agriculture, and environmental protection. Through its research and applications, chemistry provides solutions to global challenges and contributes to the improvement of human wellbeing.

Abigail Gutmann Doyle is a renowned Organic chemistry professor in Los Angeles. Her research focuses on the development of new chemical transformations in organic chemistry. She has won awards such as: Bayer Early Excellence in Science Award, Phi Lambda Upsilon National Fresenius Award, Presidential Early Career Award for Scientists and Engineers, BMS Unrestricted Grant in Synthetic Organic Chemistry.