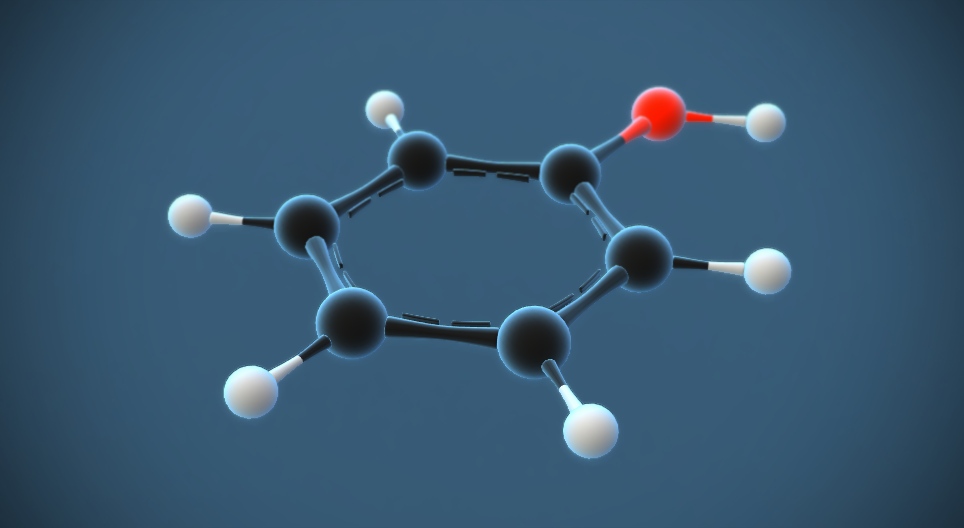
Introduction to Carbolic Acid (C₆H₅OH)
Carbolic acid, also known as phenol, is a chemical compound with the molecular formula C₆H₅OH. It is a white crystalline solid at room temperature and has a distinct, sweet, and somewhat medicinal odor. Carbolic acid is an aromatic compound, meaning it contains a benzene ring in its molecular structure, which imparts unique chemical properties.
Here are some key points about carbolic acid:
Chemical Structure: Carbolic acid’s chemical structure consists of a benzene ring (a hexagonal ring of carbon atoms with alternating single and double bonds) with a hydroxyl (-OH) group attached to one of the carbon atoms in the ring. This hydroxyl group is responsible for its classification as an alcohol.
Properties: Carbolic acid is a white crystalline solid with a melting point of around 40-42 °C. It is moderately soluble in water and forms a weakly acidic solution due to its ability to donate a proton (H+) from the hydroxyl group.
Odor: Phenol has a distinct, sweet, and somewhat medicinal odor, which can be quite strong and pungent.
Uses: Carbolic acid has various industrial and commercial applications, including:
Antiseptic: It has a long history of use as an antiseptic for disinfecting wounds and surgical instruments.
Chemical Intermediates: Phenol is used as an important intermediate chemical in the production of various compounds, such as plastics (e.g., Bakelite), resins, and dyes.
Pharmaceuticals: It is used in the synthesis of certain pharmaceuticals.
Wood Preservation: Carbolic acid has been used in wood preservation to protect against decay and insect infestation.
In the manufacture of explosives, it has been employed as a key component.
Health and Safety: Phenol can be toxic and corrosive if not handled properly. Skin contact or inhalation of its fumes can lead to irritation or health issues. Therefore, it should be used with caution and in well-ventilated areas, and appropriate safety measures should be taken.
It’s important to note that the term “carbolic acid” is often used interchangeably with “phenol,” but “phenol” is the more widely accepted and specific name for this compound in modern chemical nomenclature.
Chemical Structure and Properties of Carbolic Acid
The chemical structure and properties of carbolic acid, also known as phenol (C₆H₅OH), are as follows:
Chemical Structure:
Carbolic acid (phenol) has a unique and distinct molecular structure. It consists of a benzene ring, which is a hexagonal ring of six carbon atoms, each bonded to a hydrogen atom. In addition to the benzene ring, there is a hydroxyl (-OH) group attached to one of the carbon atoms in the ring. This hydroxyl group is responsible for its classification as an alcohol. The chemical formula is C₆H₅OH.
Properties:
Physical Properties:
State: Phenol is typically found as a white crystalline solid at room temperature. It can also appear as a liquid or a colorless to pinkish-violet crystalline mass, depending on its temperature.
Melting Point: The melting point of phenol is approximately 40-42 °C.
Odor: Phenol has a distinct, sweet, and somewhat medicinal odor. This odor is often described as pungent.
Chemical Properties:
Acidity: Phenol is a weak acid because the hydroxyl group (-OH) is capable of donating a proton (H+). This makes it slightly acidic in aqueous solutions, with a pKa value of about 10.
Solubility: Phenol is moderately soluble in water. It forms a weakly acidic solution when dissolved in water due to its ability to ionize and release H+ ions.
Reactivity: Phenol’s reactivity is influenced by the benzene ring and the hydroxyl group. It can undergo various chemical reactions, including electrophilic aromatic substitution reactions on the benzene ring.
Toxicity:
Phenol can be toxic and corrosive to living organisms. It can cause skin irritation and burns if it comes into contact with the skin.
Inhalation of phenol fumes can lead to respiratory irritation and other health issues.
Uses:
Carbolic acid (phenol) has several industrial and commercial applications, including:
Antiseptic: Historically, it has been used as an antiseptic for disinfecting wounds and surgical instruments.
Chemical Intermediates: Phenol is an important intermediate chemical used in the production of various compounds, such as plastics (e.g., Bakelite), resins, and dyes.
Pharmaceuticals: It is used in the synthesis of certain pharmaceuticals.
Wood Preservation: Phenol has been used in wood preservation to protect against decay and insect infestation.
Explosives: It is employed as a key component in the manufacture of explosives.
In summary, carbolic acid (phenol) has a distinctive chemical structure that includes a benzene ring and a hydroxyl group. It possesses both physical and chemical properties that make it useful in a range of applications, but it should be handled with care due to its potential toxicity and corrosive nature.
Synthesis and Production of Carbolic Acid
Phenol, also known as carbolic acid (C₆H₅OH), can be synthesized through various methods, and its production primarily involves the chemical conversion of starting materials containing benzene rings. Here are a few common methods for the synthesis and production of phenol:
- Cumene Process:
- The cumene process is one of the most widely used methods for the commercial production of phenol. It involves the following steps:
- Isopropylbenzene (cumene) is oxidized using air to form cumene hydroperoxide.
- Cumene hydroperoxide is then cleaved, producing phenol and acetone.
- The phenol and acetone can be separated, with phenol being the desired product.
- The cumene process is one of the most widely used methods for the commercial production of phenol. It involves the following steps:
- Chlorobenzene Hydrolysis:
- Chlorobenzene, which is a common starting material, can be hydrolyzed to form phenol. The steps involved are as follows:
- Chlorobenzene is treated with a strong base, typically sodium hydroxide (NaOH), under high-temperature conditions.
- This treatment leads to the cleavage of the chlorine atom from chlorobenzene, resulting in the formation of sodium phenoxide and sodium chloride.
- The sodium phenoxide can then be acidified to yield phenol.
- Chlorobenzene, which is a common starting material, can be hydrolyzed to form phenol. The steps involved are as follows:
- Direct Oxidation of Cyclohexene:
- In this method, cyclohexene can be oxidized to produce phenol. The process involves the following steps:
- Cyclohexene is oxidized with oxygen or air in the presence of a catalyst, often a metal catalyst such as palladium.
- The reaction leads to the formation of phenol and other byproducts, which can be separated.
- In this method, cyclohexene can be oxidized to produce phenol. The process involves the following steps:
- Hock Process:
- The Hock process is another method for the synthesis of phenol. It involves the following steps:
- Benzene is treated with an excess of chlorine gas to form chlorobenzene.
- Chlorobenzene is then hydrolyzed using a strong base, such as sodium hydroxide (NaOH), to yield phenol and sodium chloride.
- The Hock process is another method for the synthesis of phenol. It involves the following steps:
- Steam Reforming of Toluene:
- Toluene, an aromatic hydrocarbon, can be steam-reformed to produce phenol and hydrogen. The steps involved are as follows:
- Toluene is mixed with steam and heated to high temperatures.
- Under these conditions, toluene undergoes a series of reactions, leading to the formation of phenol and hydrogen gas.
- Toluene, an aromatic hydrocarbon, can be steam-reformed to produce phenol and hydrogen. The steps involved are as follows:
These are some of the common methods for the synthesis and production of phenol. The choice of method may depend on factors such as availability of starting materials, economic considerations, and the desired purity of the final product. Phenol is an important intermediate chemical with a wide range of industrial applications, making its production a significant part of the chemical industry.
Applications and Uses of Carbolic Acid in Chemistry
Carbolic acid, also known as phenol, is a widely used chemical compound in various applications in chemistry. Here are some of its common uses:
1. Antiseptic: Carbolic acid has strong antiseptic properties and is commonly used as a disinfectant. It can kill bacteria and other microorganisms, making it useful in medical and healthcare settings.
2. Chemical intermediate: Carbolic acid is an important intermediate in the production of various chemicals. It is used in the synthesis of pharmaceuticals, dyes, resins, and plastics.
3. Preservative: Phenol is used as a preservative to prevent the growth of bacteria and fungi in various products, including cosmetics, personal care products, and pharmaceuticals.
4. Solvent: Carbolic acid acts as a solvent for many organic compounds. It is widely utilized in laboratories for dissolving and extracting various substances.
5. Polymerization: Phenol is used as a starting material for the production of various polymers, such as epoxy resins, phenolic resins, and polycarbonates.
6. Industrial processes: Carbolic acid is used in several industrial processes, including the production of explosives, textiles, and synthetic fibers.
7. Laboratory reagent: Phenol is used as a reagent in various chemical reactions and analyses in laboratories. It can act as a reducing agent, acid catalyst, and reactant in many organic syntheses.
8. Wood preservative: Carbolic acid is commonly used to preserve wood and protect it from decay, insects, and fungi. It is used in the treatment of railroad ties, utility poles, and wooden structures.
9. Disinfectant: Phenol can be used as a disinfectant for surfaces, equipment, and instruments in laboratories, hospitals, and other healthcare settings.
10. Agricultural use: Carbolic acid is used in agriculture as a herbicide and pesticide. It can effectively control weed growth and kill pests on crops.
Overall, carbolic acid has a wide range of applications in chemistry due to its disinfectant, solvent, preservative, and synthetic properties. It plays a crucial role in various industries and scientific research.
Safety and Precautions when Handling Carbolic Acid
When handling carbolic acid in chemistry, it is important to take the following safety precautions:
1. Personal Protection: Wear appropriate personal protective equipment (PPE) such as gloves, goggles, and a lab coat to protect your skin and eyes from exposure.
2. Ventilation: Work in a well-ventilated area or use a fume hood to prevent inhalation of fumes or vapors. Carbolic acid can release toxic fumes when heated or in contact with certain substances.
3. Storage: Store carbolic acid in a tightly sealed container, away from heat, sunlight, and incompatible substances. Keep it in a cool and dry place.
4. Handling: Always handle carbolic acid with extreme caution. Use appropriate tools, such as glass pipettes, to avoid direct contact with the substance. Avoid splashing or spilling the acid.
5. Dilution: In case of dilution, add the acid slowly to water with vigorous stirring. Never pour water into carbolic acid as it can cause splashing and release of heat.
6. First Aid: In case of accidental exposure, immediately rinse the affected area with plenty of water for at least 15 minutes. Remove any contaminated clothing. Seek medical attention promptly.
7. Spill Response: In case of a spill, use appropriate spill control measures, such as absorbent materials like sand or vermiculite, to contain and neutralize the acid. Dispose of the waste properly according to local regulations.
8. Emergency Preparedness: Know the location of emergency safety equipment, such as eyewash stations, and fire extinguishers. Familiarize yourself with the appropriate procedures for handling chemical spills or accidents.
9. Labeling: Always ensure that the container holding carbolic acid is properly labeled with the name of the substance, hazard warnings, and necessary precautionary information.
Remember to consult the safety data sheet (SDS) provided by the manufacturer for specific handling guidelines and safety information for carbolic acid.

Abigail Gutmann Doyle is a renowned Organic chemistry professor in Los Angeles. Her research focuses on the development of new chemical transformations in organic chemistry. She has won awards such as: Bayer Early Excellence in Science Award, Phi Lambda Upsilon National Fresenius Award, Presidential Early Career Award for Scientists and Engineers, BMS Unrestricted Grant in Synthetic Organic Chemistry.