Introduction to Epinephrine
Epinephrine, also known as adrenaline, is a hormone and neurotransmitter belonging to a class of compounds called catecholamines. It plays a significant role in the body’s response to stress and emergency situations. Chemically, epinephrine is classified as a sympathomimetic amine due to its ability to stimulate the sympathetic nervous system.
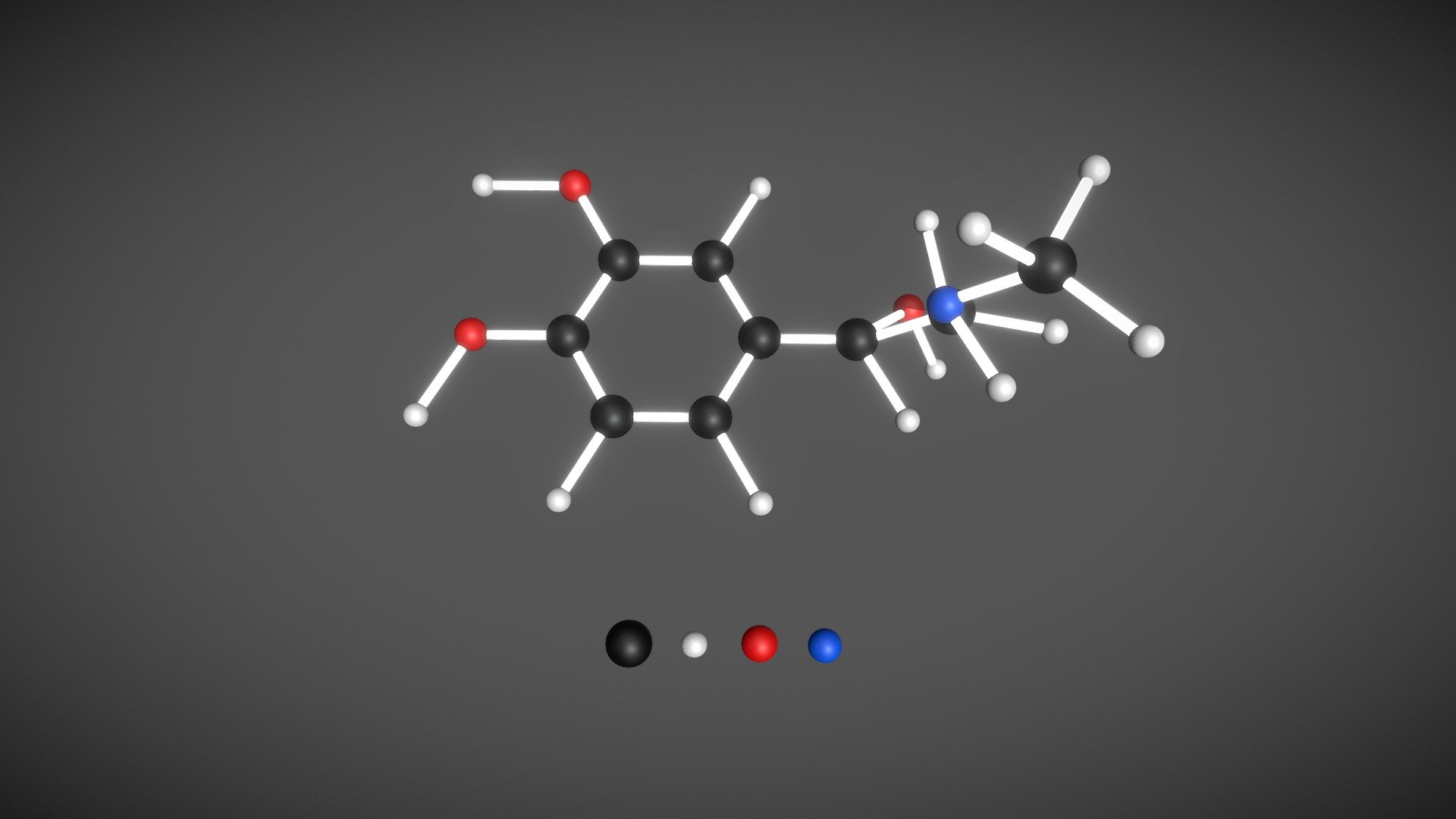
The chemical structure of epinephrine consists of a benzene ring, with two substituents – a hydroxyl group (-OH) and an amino group (-NH2) – attached to the benzene ring. The hydroxyl group is found at the meta position, while the amino group is located at the para position.
The molecular formula of epinephrine is C9H13NO3, and its systematic name is (R)-4-(1-Hydroxy-2-(methylamino)ethyl)benzene-1,2-diol. It has a molecular weight of approximately 183 grams per mole.
Epinephrine is primarily produced in the adrenal glands, which are located on top of the kidneys. It is released into the bloodstream during stressful situations, causing a range of physiological responses. These responses include increased heart rate and blood pressure, dilation of blood vessels, increased blood flow to muscles, and the release of glucose from the liver. All of these effects are part of the body’s “fight-or-flight” response.
In medicine, epinephrine is widely used as a medication for the treatment of severe allergic reactions, such as anaphylaxis. It is administered by injection and acts as a potent bronchodilator, vasoconstrictor, and cardiac stimulant. Epinephrine can quickly reverse the symptoms of an allergic reaction, such as difficulty breathing, swelling, and low blood pressure, by restoring normal physiological functions.
Epinephrine also has applications outside of medicine. It is used in chemical analysis as a reagent and is commonly employed in chromatography to separate and identify various compounds. Additionally, epinephrine is used in sports medicine as a performance-enhancing drug due to its stimulatory effects on the body.
In conclusion, epinephrine is a vital compound in the human body, serving as a hormone and neurotransmitter involved in the body’s response to stress. Its chemical structure and properties enable it to elicit various physiological responses, making it a crucial tool in medicine and other fields.
Chemical Formula of Epinephrine
The chemical formula of Epinephrine is C9H13NO3
Structure of Epinephrine
The structure of epinephrine, also known as adrenaline, is a complex molecule with several functional groups. Its chemical formula is C9H13NO3.
Epinephrine consists of a catechol ring, which is a benzene ring with two hydroxyl groups (-OH) attached at the meta position (positions 1 and 3) to form a 1,2-dihydroxybenzene structure.
Attached to the catechol ring is an ethylamine side chain that contains an amino group (-NH2) at one end and an ethyl group (-C2H5) at the other end. The amino group is attached to the carbon atom at position 1 (ortho to the hydroxyl groups) of the benzene ring.
At position 2 (para to the hydroxyl groups) of the benzene ring, there is a secondary alcohol group (-OH) attached.
Lastly, at position 3 (meta to the ethyl group), there is a primary alcohol group (-OH) attached.
Overall, the structure of epinephrine consists of a catechol ring with a hydroxyl group at position 2 and amino and ethyl groups attached at position 1, creating a unique and important molecule in physiology and pharmacology.
Properties of Epinephrine
Epinephrine, also known as adrenaline, is a hormone and neurotransmitter that plays an essential role in the human body’s response to stress. In chemistry, epinephrine possesses several important properties:
1. Chemical structure: Epinephrine is a catecholamine, with a chemical formula of C9H13NO3. It contains a catechol ring and an amine group, making it an amine.
2. Water solubility: Epinephrine is highly soluble in water due to the presence of hydrophilic hydroxyl (OH) and amine (NH2) groups. This property allows it to be transported in the bloodstream.
3. pH-dependent stability: Epinephrine is relatively stable in acidic conditions but becomes less stable at alkaline pH. In basic solutions, epinephrine oxidizes easily, resulting in the formation of brown-colored degradation products.
4. Oxidation and reduction reactions: Epinephrine can undergo oxidation reactions in the presence of enzymes or atmospheric oxygen. It is oxidized to various products, including adrenochrome, which contributes to the brown coloration often associated with the degradation of epinephrine-containing solutions. On the other hand, epinephrine can be reduced to its inactive form, epinephrine-β-ol, through the addition of reducing agents like sodium bisulfite.
5. Chirality: Epinephrine is a chiral molecule, meaning it exists in two optically active forms: D-epinephrine and L-epinephrine. The D form is the biologically active enantiomer, responsible for the physiological effects in the human body.
6. Biological activity: Epinephrine acts as a potent agonist for adrenergic receptors, particularly α- and β-adrenergic receptors. It stimulates the sympathetic nervous system, triggering the fight-or-flight response and various physiological effects, such as increased heart rate, blood pressure, and respiratory rate.
7. Medical applications: Epinephrine finds wide application in medicine, particularly in emergency situations such as anaphylaxis or cardiac arrest. It is administered via injection to rapidly counteract severe allergic reactions or to initiate cardiopulmonary resuscitation (CPR).
Overall, the properties of epinephrine make it a critical compound in both chemistry and biology, contributing to its significant role in the human body’s regulation of various physiological processes.
Applications of Epinephrine
Epinephrine, also known as adrenaline, has several important applications in medicine and other fields. Some of the key applications of epinephrine include:
Treatment of Anaphylaxis: Epinephrine is commonly used to treat severe allergic reactions, known as anaphylaxis. It helps to reverse the symptoms of anaphylaxis, such as difficulty breathing, swelling, and a drop in blood pressure, by constricting blood vessels and opening the airways.
Cardiac Arrest: Epinephrine is used in emergency situations to treat cardiac arrest. It stimulates the heart and helps restore a regular heartbeat when administered as part of advanced cardiac life support (ACLS) protocols.
Asthma: Epinephrine is sometimes used in the treatment of acute asthma attacks, as it can relax the airway smooth muscles, making it easier to breathe.
Local Anesthesia: Epinephrine is often added to local anesthetics like lidocaine to prolong their effects. It constricts blood vessels, reducing blood flow at the injection site, which slows down the absorption of the anesthetic, leading to longer pain relief.
Hemostasis: In surgical and medical procedures, epinephrine is sometimes used to control bleeding. It constricts blood vessels, reducing blood flow to the site of bleeding and making it easier for healthcare professionals to manage bleeding.
Hypotension: Epinephrine can be administered to patients with severely low blood pressure, especially in emergency situations, to raise blood pressure and improve blood flow to vital organs.
As a Vasopressor: Epinephrine is used in critical care settings to support blood pressure in patients with shock or cardiac arrest. It acts as a vasopressor, constricting blood vessels and increasing cardiac output.
Myocardial Infarction: In some cases, epinephrine may be used during a myocardial infarction (heart attack) to improve blood flow to the heart muscle.
Bronchodilation: Epinephrine can be used to dilate the bronchial airways, making it easier for people with respiratory conditions such as chronic obstructive pulmonary disease (COPD) to breathe.
Allergic Reactions in Dentistry: Dentists may use epinephrine in local anesthetics to reduce bleeding during oral procedures.
It’s important to note that while epinephrine can be life-saving in many situations, it should only be administered by trained medical professionals, as improper use can lead to complications and side effects.

Abigail Gutmann Doyle is a renowned Organic chemistry professor in Los Angeles. Her research focuses on the development of new chemical transformations in organic chemistry. She has won awards such as: Bayer Early Excellence in Science Award, Phi Lambda Upsilon National Fresenius Award, Presidential Early Career Award for Scientists and Engineers, BMS Unrestricted Grant in Synthetic Organic Chemistry.