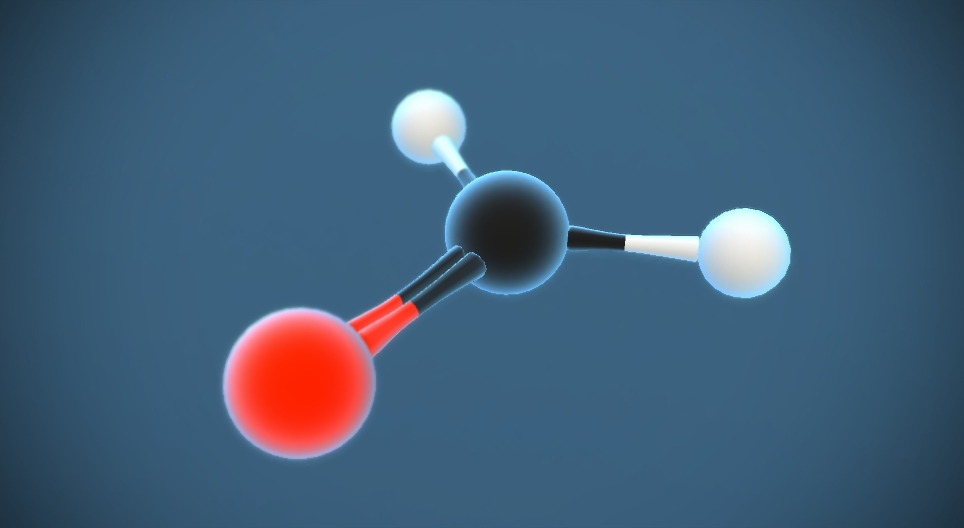
Definition of Formaldehyde (CH₂O) in chemistry
Formaldehyde (CH₂O) is a colorless, pungent-smelling gas that is the simplest aldehyde. It is a highly reactive compound composed of one carbon atom, two hydrogen atoms, and one oxygen atom. Formaldehyde is widely used in various industries and research laboratories as a disinfectant, preservative, and fixative. It is also an important starting material for the production of many chemicals and materials, including plastics, resins, and textiles. In addition, formaldehyde is a byproduct of incomplete combustion and can be found in cigarette smoke and automobile exhaust. It is classified as a carcinogen and can cause both short-term and long-term health effects, such as respiratory issues and allergies, if exposure occurs at high concentrations.
Physical and chemical properties of formaldehyde
Physical properties of formaldehyde:
1. Formaldehyde has a pungent and choking odor.
2. It has a boiling point of -19°C (-2°F) and a melting point of -92°C (-133.6°F).
3. It is a colorless gas at room temperature but can be obtained as a liquid state by cooling or under high pressure.
4. The density of formaldehyde gas is 0.815 g/cm³, while its liquid form has a density of 0.815 g/mL.
5. It is highly soluble in water, with a solubility of approximately 550 g/L at room temperature.
Chemical properties of formaldehyde:
1. Formaldehyde undergoes polymerization reactions, forming paraformaldehyde, a solid polymer made up of repeating formaldehyde units.
2. It can react with various nucleophiles, such as amines and alcohols, to form compounds known as imines and acetals respectively.
3. Formaldehyde can undergo oxidation reactions, converting to formic acid or carbon dioxide, depending on the reaction conditions.
4. It can act as a reducing agent, donating electrons to other substances in redox reactions.
5. Formaldehyde is highly reactive towards organic compounds, particularly those containing double bonds or functional groups such as alcohols, amines, and thiols. It can undergo addition reactions with these compounds, forming products such as aldehydes, imines, and thioacetals.
Production and industrial applications of formaldehyde
Formaldehyde is widely used in various production and industrial applications in chemistry. Some of its major uses include:
1. Production of Resins: Formaldehyde is a key component in the production of a wide range of resins, including urea-formaldehyde resins, phenol-formaldehyde resins, and melamine-formaldehyde resins. These resins are used in the manufacturing of plywood, particleboard, laminates, and coatings due to their excellent adhesive properties.
2. Textile Industry: Formaldehyde is used in the textile industry as a cross-linking agent for improving the wrinkle resistance and durability of fabrics. It is commonly used in the production of permanent press fabrics and clothing.
3. Production of Plastics: Formaldehyde is a building block for the production of various plastics, such as polyoxymethylene (POM), which is used in the manufacturing of gears, bearings, and other precision parts. It is also used in the production of polyurethane foams, which find wide applications in the automotive, furniture, and construction industries.
4. Disinfectant: Due to its strong antimicrobial properties, formaldehyde is used as a disinfectant and preservative for medical equipment, laboratories, and mortuaries. Its ability to kill bacteria, viruses, and fungi makes it an effective sterilizing agent.
5. Agricultural Applications: Formaldehyde is used in agricultural chemistry as a soil fumigant to control nematodes and other pests. It is also used as a disinfectant in animal husbandry and for preserving animal specimens.
6. Production of Dyes: Formaldehyde is used in the manufacturing of some dyes and pigments. It acts as a reducing agent and plays a crucial role in the color development process.
7. Environmental Applications: Formaldehyde is used in the treatment of wastewater and air pollution control systems. It can effectively remove various pollutants, such as hydrogen sulfide, from industrial emissions.
It is worth mentioning that formaldehyde’s use in some applications, such as in textiles and resins, has raised concerns due to its potential health hazards. Therefore, careful handling and appropriate safety measures are essential in these industrial applications.
Health and environmental concerns associated with formaldehyde
Formaldehyde, a colorless gas with a pungent odor, is widely used in various industries, including chemistry. While it has many industrial applications, formaldehyde poses health and environmental concerns.
Health concerns:
1. Respiratory issues: Formaldehyde is a known respiratory irritant and can cause irritation of the nose, throat, and lungs. Prolonged exposure or high levels of formaldehyde may lead to coughing, wheezing, and difficulty breathing.
2. Allergic reactions: Some individuals may develop allergic reactions to formaldehyde, which can manifest as skin rashes, itching, and dermatitis. People with pre-existing respiratory conditions or asthma may experience exacerbated symptoms when exposed to formaldehyde.
3. Carcinogenicity: Formaldehyde is classified as a Group 1 human carcinogen by the International Agency for Research on Cancer (IARC). Prolonged and repeated exposure to formaldehyde has been linked to an increased risk of nasopharyngeal cancer and leukemia.
4. Occupational hazards: Workers in industries that use or produce formaldehyde, such as manufacturing, laboratories, and healthcare facilities, may face occupational hazards. Proper safety precautions, such as using personal protective equipment and ventilation systems, are necessary to minimize exposure and prevent health risks.
Environmental concerns:
1. Air pollution: Formaldehyde emissions from industrial sources, such as chemical plants and manufacturing facilities, can contribute to air pollution. It is a volatile organic compound (VOC) that reacts with other pollutants, contributing to the formation of ground-level ozone and smog.
2. Water contamination: Improper disposal of formaldehyde-containing waste or runoff from industrial facilities can potentially contaminate water sources. Formaldehyde is toxic to aquatic life and can negatively impact ecosystems.
3. Soil contamination: Spills or leaks of formaldehyde can lead to soil contamination. Formaldehyde has the potential to affect soil fertility and disrupt the natural balance of microorganisms in the soil.
4. Bioaccumulation: Formaldehyde has the ability to bioaccumulate, meaning it can accumulate in the tissues of living organisms over time. This can lead to an increased concentration of formaldehyde in the food chain, potentially posing risks to human and animal health.
To mitigate the health and environmental concerns associated with formaldehyde, regulatory bodies enforce guidelines and regulations on its production, use, and disposal. Strict adherence to safety protocols, proper handling, and suitable waste management practices are essential in reducing the impact of formaldehyde on both human health and the environment.
Regulations and safety measures for handling formaldehyde
Handling formaldehyde in a chemistry laboratory requires adherence to specific regulations and safety measures to ensure the well-being of laboratory personnel. These include:
1. Regulatory Compliance: Formaldehyde is classified as a hazardous substance by various regulatory bodies, such as the Occupational Safety and Health Administration (OSHA) in the United States. Follow all relevant regulations and guidelines, including proper storage, handling, and disposal of formaldehyde.
2. Personal Protective Equipment (PPE): Wear appropriate PPE, including but not limited to, gloves, lab coats, safety goggles, and respiratory protection, when working with formaldehyde. This helps prevent direct contact with the chemical and inhalation of its vapors.
3. Ventilation: Perform all formaldehyde-related work in a well-ventilated area or under a fume hood. Adequate ventilation helps to minimize the concentration of formaldehyde vapors in the air, reducing the risk of inhalation.
4. Storage: Store formaldehyde in approved containers, away from incompatible substances, such as strong acids. Keep containers tightly closed when not in use. Furthermore, it is important to label the containers properly, indicating the name of the chemical and any hazards associated with it.
5. Spill and Leak Response: Formaldehyde spills should be promptly and safely cleaned up using absorbent materials. Avoid creating aerosols or spreading the spill further. For larger spills or situations where the spill cannot be controlled, evacuate the area and contact an appropriate authority (e.g., hazardous materials response team) for assistance.
6. Waste Disposal: Dispose of formaldehyde waste according to applicable regulations. Follow institutional protocols for disposing of formaldehyde-contaminated materials and solutions, and never dispose of formaldehyde down the drain.
7. Risk Assessment and Training: Conduct a thorough risk assessment before working with formaldehyde. Provide adequate training to laboratory personnel regarding the hazards, safe handling techniques, and emergency procedures associated with formaldehyde.
8. Emergency Preparedness: Have appropriate emergency response measures in place, including access to emergency eyewash stations, safety showers, and spill kits. Ensure that all laboratory personnel are aware of the emergency procedures and know how to respond in case of a formaldehyde-related incident.
9. Proper Monitoring: Regularly monitor formaldehyde concentrations in the laboratory air using appropriate monitoring equipment. This helps ensure that exposure levels are within acceptable limits and allows for timely corrective actions if necessary.
Remember, this list is not exhaustive, and it is essential to consult relevant regulations, guidelines, and best practices specific to your jurisdiction and institutional requirements when handling formaldehyde in a chemistry laboratory.

Abigail Gutmann Doyle is a renowned Organic chemistry professor in Los Angeles. Her research focuses on the development of new chemical transformations in organic chemistry. She has won awards such as: Bayer Early Excellence in Science Award, Phi Lambda Upsilon National Fresenius Award, Presidential Early Career Award for Scientists and Engineers, BMS Unrestricted Grant in Synthetic Organic Chemistry.