Introduction to Formalin
Formalin is a solution of formaldehyde gas in water, and it is commonly used for various purposes, including as a disinfectant, preservative, and fixative in laboratory and medical settings. Here’s an introduction to formalin, covering its composition, uses, and potential health and safety considerations:
Composition:
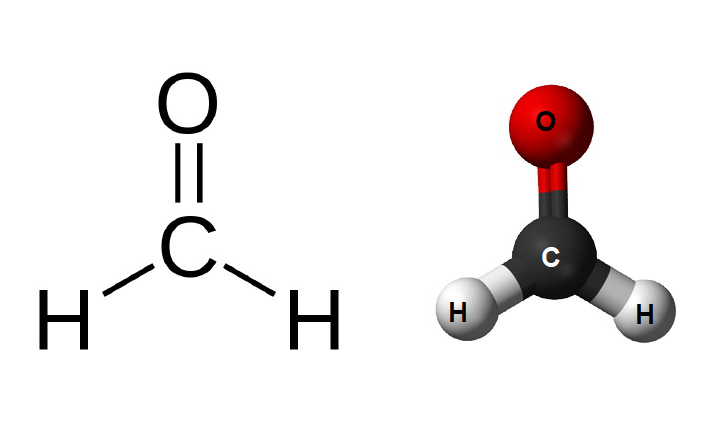
Formalin primarily consists of formaldehyde (CH2O), which is a colorless and strong-smelling gas that is highly reactive. To make formalin, formaldehyde gas is dissolved in water to create a solution with a specific formaldehyde concentration. The concentration can vary depending on the intended use, but it is typically between 37% and 50% formaldehyde by weight.
Uses:
Preservative: Formalin is widely used to preserve biological specimens, such as tissues and organs, for research and diagnostic purposes. This fixation process prevents decay and decomposition, allowing for the study of tissues under a microscope.
Disinfectant: Formalin’s strong antibacterial properties make it suitable for disinfecting equipment, surfaces, and environments. It is used in laboratories, healthcare facilities, and the funeral industry.
Tanning: Formalin is also used in leather tanning to preserve animal hides and create durable leather products.
Manufacturing: It plays a role in the production of various products, including adhesives, resins, and plastics.
Health and Safety Considerations:
Toxicity: Formalin is toxic when inhaled, ingested, or absorbed through the skin. Prolonged or high-level exposure to formaldehyde can lead to health issues, including respiratory problems, eye and skin irritation, and in severe cases, cancer. Adequate ventilation and protective equipment, such as gloves and goggles, are essential when working with formalin.
Proper Storage: Formalin should be stored in a well-ventilated area away from incompatible substances. It should be kept in containers with proper labeling to prevent accidents.
Disposal: Disposing of formalin and formalin-treated specimens must be done following specific regulations and guidelines to prevent environmental contamination.
Regulations:
Many countries and regions have established regulations and guidelines governing the use, handling, and disposal of formalin due to its potential health and environmental hazards. It’s crucial to adhere to these regulations to ensure the safe and responsible use of formalin.
In summary, formalin is a solution of formaldehyde in water, and it finds widespread use as a preservative, disinfectant, and fixative in various applications. However, its toxicity necessitates strict safety measures and compliance with regulations to protect the health of individuals and the environment.
Chemical Properties of Formalin (HCHO)
Formalin, which is a solution of formaldehyde (HCHO) gas in water, exhibits various chemical properties associated with formaldehyde itself. Here are some of the important chemical properties of formalin (HCHO):
Reactivity: Formaldehyde is a highly reactive compound. It readily undergoes chemical reactions with a wide range of compounds and functional groups, making it a versatile reagent in organic chemistry.
Electrophilic Nature: Formaldehyde is an electrophile, meaning it is electron-deficient and seeks electrons from other molecules. This property makes it a good reactant in nucleophilic addition reactions.
Polymerization: Formaldehyde can polymerize, forming paraformaldehyde or trioxane, which are solid, high molecular weight polymers. These substances are used in various industrial applications, including as a source of formaldehyde for controlled release.
Reduction: Formaldehyde can be reduced to methanol (CH3OH) through the addition of hydrogen. This reduction reaction is often used in the production of methanol.
Aldol Condensation: Formaldehyde can undergo aldol condensation reactions with itself or other carbonyl compounds, yielding various compounds with multiple carbon-carbon bonds.
Oxidation: Formaldehyde can be further oxidized to formic acid (HCOOH), which is an important intermediate in various chemical processes.
Derivatives: Formaldehyde can be used as a starting material to synthesize a wide range of chemical derivatives, such as methylene glycol, paraformaldehyde, and resins like urea-formaldehyde and phenol-formaldehyde.
Cross-linking Agent: Formalin is widely used as a cross-linking agent for proteins in various biological and chemical applications. It forms covalent bonds with amino groups, making it useful in preserving and stabilizing biological samples for research.
Dehydration: Formaldehyde can act as a dehydrating agent in certain reactions, such as the conversion of primary alcohols to alkenes through the formation of hemiacetals.
Acidity: Although formaldehyde is not a strong acid, it can donate a proton (H+) to a base in solution, forming the hydroxymethanium ion (H2COH+). This property can be relevant in certain chemical reactions.
Carbonyl Group: Formaldehyde contains a carbonyl group (C=O), which makes it susceptible to nucleophilic attack at the carbon atom. This carbonyl group is the basis for many of its chemical reactivities.
It’s important to note that formaldehyde is a toxic and volatile compound, and its handling and use should be done with care, following safety guidelines and regulations to ensure the well-being of individuals and the environment.
Uses of Formalin in Chemistry
Formalin, which is a solution of formaldehyde gas (HCHO) in water, has several important uses in chemistry. Its reactivity and ability to form covalent bonds make it a versatile reagent in various chemical processes. Here are some of the common uses of formalin in chemistry:
Preservative and Fixative: Formalin is frequently used in the preservation of biological specimens, including tissues, organs, and cells. It acts as a fixative, preventing decay and decomposition of the specimens, allowing for their long-term storage and microscopic examination.
Cross-linking Agent: In biochemistry and molecular biology, formalin is employed as a cross-linking agent to stabilize and study protein-protein or protein-nucleic acid interactions. It forms covalent bonds with amino groups in proteins, helping to preserve the structure of macromolecules for research purposes.
Dehydration Agent: Formalin can act as a dehydrating agent in certain chemical reactions. It is used to remove water from samples or to facilitate reactions involving water-sensitive compounds.
Reagent in Organic Synthesis: Formaldehyde is a versatile reagent in organic chemistry. It is used in various reactions, including the synthesis of methylene bridges in organic molecules, the formation of acetals and hemiacetals, and the preparation of different derivatives and intermediates.
Aldol Condensation: Formaldehyde can participate in aldol condensation reactions, especially when it reacts with itself. This reaction is a fundamental step in the formation of a variety of organic compounds, including linear and cyclic compounds.
Reduction to Methanol: Formalin can be reduced to methanol (CH3OH) through the addition of hydrogen. This reduction is significant in the production of methanol, which is used as a solvent and as a feedstock in the chemical industry.
Polymerization: Formaldehyde can polymerize to form paraformaldehyde or trioxane, which are solid, high molecular weight polymers. These polymerization products have various industrial applications, including as sources of formaldehyde in controlled release.
Production of Resins: Formalin is used in the synthesis of formaldehyde-based resins, such as urea-formaldehyde and phenol-formaldehyde resins. These resins are essential in the manufacture of adhesives, laminates, and molded products.
Oxidation to Formic Acid: Formaldehyde can be further oxidized to form formic acid (HCOOH), an important intermediate in various chemical processes.
Intermediate in Organic Reactions: Formalin serves as an intermediate in numerous chemical reactions, particularly those that involve the introduction of a carbonyl group into organic molecules.
These are just a few of the many uses of formalin in chemistry. It plays a crucial role in various research, industrial, and laboratory applications, thanks to its reactivity and ability to form covalent bonds with a wide range of organic and biological compounds.
Health and Safety Concerns associated with Formalin
Formalin, which is a solution of formaldehyde gas (HCHO) in water, poses several health and safety concerns due to its toxic nature. It’s essential to handle and use formalin with care to minimize the associated risks. Here are the primary health and safety concerns associated with formalin:
Toxicity: Formalin is highly toxic and can cause a range of health problems, particularly upon inhalation, ingestion, or skin contact. Prolonged or high-level exposure can lead to acute and chronic health issues, including:
Respiratory irritation and problems, such as coughing, wheezing, and bronchitis.
Eye and skin irritation, which can manifest as redness, itching, and dermatitis.
Headaches and dizziness.
Allergic reactions, including sensitization to formaldehyde.
Potential carcinogenicity, with long-term exposure associated with an increased risk of certain cancers, such as nasopharyngeal and leukemia.
Adequate Ventilation: To mitigate inhalation risks, areas where formalin is used should have proper ventilation systems to remove formaldehyde vapors and ensure a safe working environment.
Personal Protective Equipment (PPE): It’s essential for individuals working with formalin to wear appropriate PPE, which may include safety goggles, gloves, lab coats, and, in some cases, respiratory protection. This helps prevent skin and eye contact and inhalation of formaldehyde fumes.
Proper Storage and Labeling: Formalin should be stored in well-ventilated areas away from incompatible substances. Containers should be labeled accurately to prevent accidents or mishandling.
Spill Response: Spills of formalin should be handled with caution. Spill response procedures should be in place, and appropriate absorbent materials, protective gear, and disposal methods should be used.
Emergency Equipment: Facilities using formalin should be equipped with emergency eyewash stations and safety showers to provide immediate decontamination in case of accidental exposure.
Regulatory Compliance: Formalin use and disposal should adhere to regulations and guidelines established by local, national, and international authorities to protect both human health and the environment.
Training and Education: Workers should be adequately trained on the safe handling, storage, and disposal of formalin. They should also be aware of the associated risks and symptoms of exposure.
Biological Samples: When using formalin as a fixative or preservative for biological specimens, it’s crucial to follow proper procedures and use appropriate containment systems to prevent formalin exposure.
Waste Disposal: Proper disposal of formalin and formalin-treated materials is essential to prevent environmental contamination. Waste should be managed in compliance with hazardous waste regulations.
Monitoring: Regular monitoring of formaldehyde levels in the workplace can help ensure that exposure limits are not exceeded and that control measures are effective.
In summary, formalin presents various health and safety concerns due to its toxic and irritating properties. Careful handling, appropriate protective measures, and compliance with regulations are crucial to minimize the associated risks and create a safe working environment when using formalin in laboratory, medical, or industrial applications.
Conclusion
In conclusion, chemistry is a fundamental science that plays a crucial role in understanding the composition, structure, properties, and behaviors of matter. It provides a foundation for numerous fields such as medicine, materials science, environmental science, and energy production. Through various theories, principles, and laboratory techniques, chemists are able to manipulate and transform matter, leading to advancements in technology, improved health outcomes, and a deeper understanding of the natural world. Chemistry continues to be a dynamic and evolving discipline, with ongoing research and discoveries shaping our understanding of the universe at both the macroscopic and microscopic levels.

Abigail Gutmann Doyle is a renowned Organic chemistry professor in Los Angeles. Her research focuses on the development of new chemical transformations in organic chemistry. She has won awards such as: Bayer Early Excellence in Science Award, Phi Lambda Upsilon National Fresenius Award, Presidential Early Career Award for Scientists and Engineers, BMS Unrestricted Grant in Synthetic Organic Chemistry.