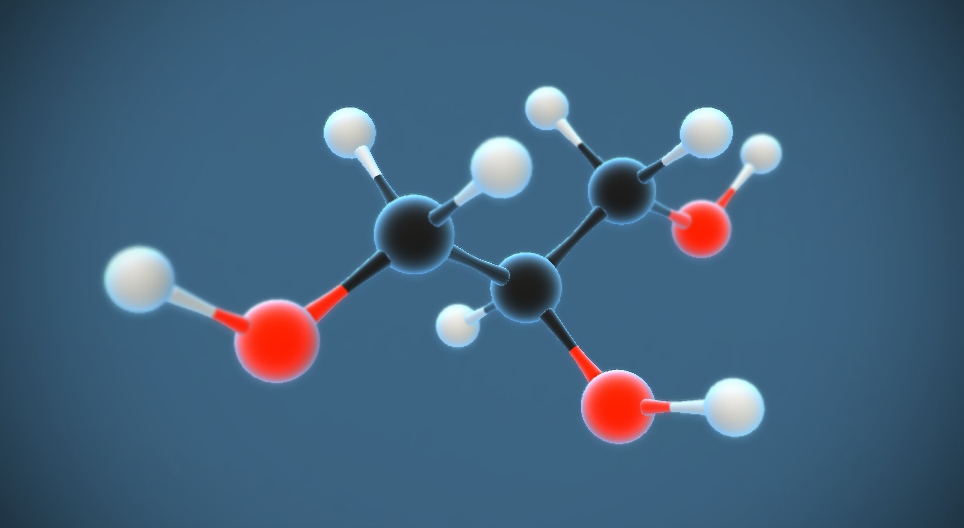
Introduction to Glycerol
Glycerol is a compound that plays a significant role in chemistry. It is a type of alcohol with three hydroxyl (OH) groups attached to a three-carbon chain. Its chemical formula is C3H8O3.
Glycerol is a colorless, odorless, and syrupy liquid that is highly soluble in water. It is commonly known as glycerin or glycerine. It has a sweet taste and is widely used as a solvent, a moisturizer, and a thickening agent in various industries.
One of the most important properties of glycerol is its ability to mix with both polar and non-polar substances, making it a versatile compound. It is hygroscopic, meaning it can absorb and retain moisture from the surrounding environment. Due to this property, it is commonly used in the production of cosmetics, pharmaceuticals, and food products.
Glycerol has a wide range of applications in the chemical industry. It is utilized in the production of explosives, as a solvent for dyes and chemicals, and as an ingredient in the manufacturing of plastics and polymers. It is also used as a precursor for the synthesis of various compounds, such as esters and ethers.
Additionally, glycerol is important in biochemistry. It is a crucial component of lipids, which are an essential part of cell membranes. Glycerol can be converted into glucose through a process called gluconeogenesis, providing energy to the body in times of fasting or low-carbohydrate intake.
In summary, glycerol is a versatile compound that has numerous applications in various industries. Its unique properties, such as solubility, hygroscopicity, and reactivity, make it an essential component in the production of various materials and substances. Its significance extends to biochemistry, where it plays a crucial role in cellular processes and energy metabolism.
Chemical Formula of Glycerol (C₃H₈O₃)
The chemical formula of glycerol is C3H8O3.
Properties of Glycerol
Glycerol, also known as glycerin or glycerine, is a sweet-tasting, viscous liquid that is widely used in various industries. It possesses several important properties in chemistry, including:
1. Chemical Formula: The chemical formula of glycerol is C3H8O3, indicating that it contains three carbon atoms, eight hydrogen atoms, and three oxygen atoms.
2. Physical State: Glycerol is a colorless, odorless liquid at room temperature. It has a high boiling point of 290 degrees Celsius, indicating its relatively high stability.
3. Solubility: Glycerol is highly soluble in water. It can mix well with polar solvents due to its polar hydroxyl (-OH) groups. However, it is only sparingly soluble in non-polar solvents like hexane or ether.
4. Hygroscopic Nature: Glycerol is hygroscopic, meaning it has a tendency to absorb moisture from the atmosphere. This property makes it a humectant, widely used in moisturizers and skincare products.
5. Viscosity: Glycerol is a highly viscous liquid, meaning it has a thick consistency. This property makes it useful in various applications, such as in the pharmaceutical and cosmetics industries.
6. Polyol Functionality: Glycerol is a polyol, meaning it contains multiple hydroxyl (-OH) groups. It has three hydroxyl groups, which makes it reactive and serves as the basis for many of its chemical applications.
7. Non-Toxic and Non-Flammable: Glycerol is non-toxic, making it safe for use in various applications, including as a food additive, pharmaceutical ingredient, and skincare product. It is also non-flammable, further enhancing its safety.
8. Biological Uses: Glycerol is widely employed in biology and biochemistry. It is commonly used as a cryoprotectant, allowing biological samples and cells to be preserved at low temperatures without damage.
9. Chemical Reactivity: Glycerol can undergo various chemical reactions, such as oxidation, esterification, and dehydration. These reactions allow the synthesis of various compounds, such as fatty acids, esters, and nitroglycerin.
10. Renewable and Sustainable Source: Glycerol can be derived from plant and animal fats, making it a renewable and sustainable resource. It is produced on a large scale during the production of biodiesel, providing a valuable byproduct that can be utilized in various applications.
Uses of Glycerol in Chemistry
Glycerol, also known as glycerin, is a commonly used chemical compound in various fields of chemistry. Some important uses of glycerol in chemistry include:
1. Solvent: Glycerol is a versatile solvent and is used to dissolve a wide range of substances, including organic compounds, inorganic salts, and polymers. It is particularly useful as a solvent for hydrophilic (water-soluble) compounds.
2. Catalyst: Glycerol can act as a catalyst in certain chemical reactions. For example, it can be used as a catalyst in the synthesis of acrolein from glycerol through dehydration.
3. Stabilizer: Glycerol is often used as a stabilizer in various chemical formulations. It helps prevent the decomposition or degradation of certain substances, such as drugs, food additives, and cosmetic products.
4. Humectant: Glycerol has excellent water-retaining properties and is commonly used as a humectant in many industries. It helps to retain moisture and prevent drying out of substances, such as pharmaceuticals, cosmetics, and food products.
5. Intermediate in chemical synthesis: Glycerol is a versatile building block in organic chemistry. It serves as an important intermediate for the synthesis of various compounds, including polyols, esters, and surfactants.
6. Polymer production: Glycerol is used as a raw material in the production of various polymers, such as polyurethanes and polyesters. It can be polymerized with other monomers to form high molecular weight compounds with desired properties.
7. Viscosity modifier: Glycerol is often added to various liquids, such as lubricants and hydraulic fluids, to modify their viscosity. It helps to improve the flow properties and reduce friction.
8. Cryoprotectant: Glycerol is commonly used as a cryoprotectant in biological and biochemical applications. It is added to cell and tissue samples to protect them from damage during freezing and thawing processes.
These are just a few examples of the many uses of glycerol in chemistry. Its unique properties make it a valuable compound in numerous applications across various industries.
Conclusion
In conclusion, chemistry is a fundamental science that plays a crucial role in our everyday lives. It helps us understand the composition, properties, and interactions of matter, leading to advancements in various fields such as medicine, materials science, environmental science, and many more. Chemistry provides a framework for explaining the natural phenomena around us and helps us develop new technologies and innovations to improve our quality of life. By studying chemistry, we can better understand the world we live in and make informed decisions about issues related to our health, environment, and sustainability.

Abigail Gutmann Doyle is a renowned Organic chemistry professor in Los Angeles. Her research focuses on the development of new chemical transformations in organic chemistry. She has won awards such as: Bayer Early Excellence in Science Award, Phi Lambda Upsilon National Fresenius Award, Presidential Early Career Award for Scientists and Engineers, BMS Unrestricted Grant in Synthetic Organic Chemistry.