Introduction to Methane (CH₄)
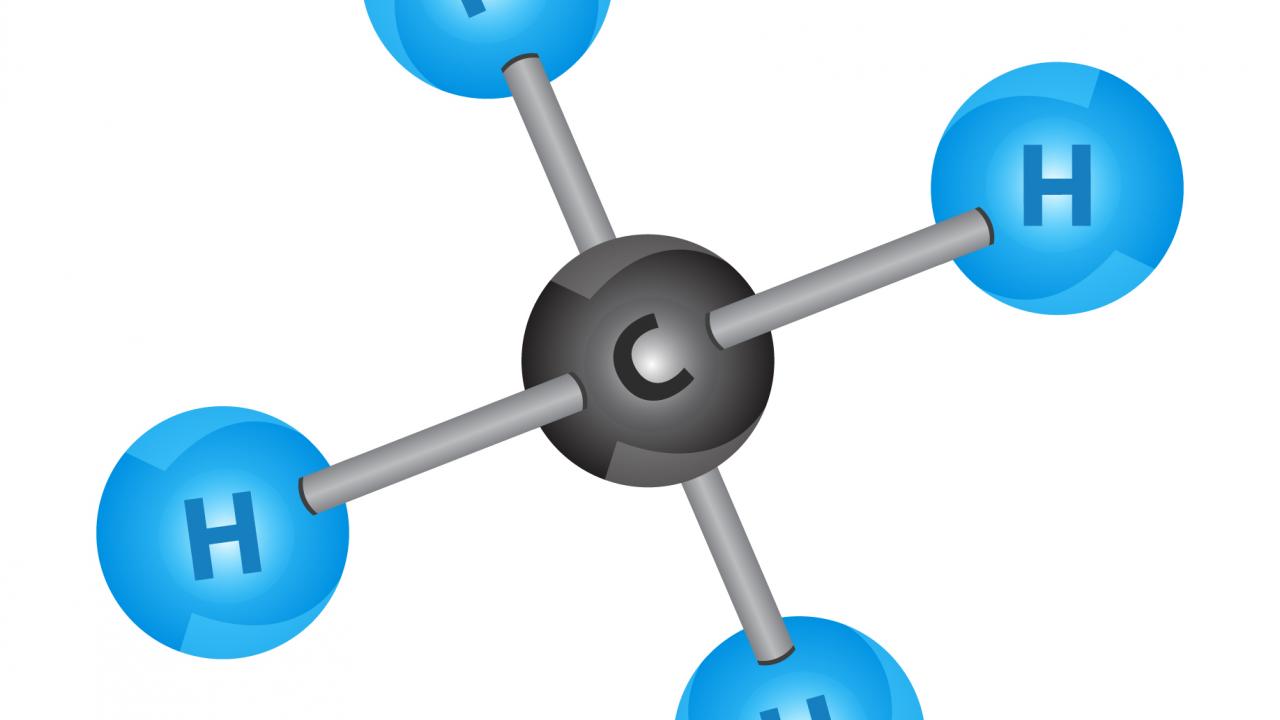
Methane, chemically represented as CH₄, is a simple hydrocarbon compound that consists of one carbon atom bonded to four hydrogen atoms. It is the primary component of natural gas and is the simplest member of the alkane family.
Methane is a colorless and odorless gas at normal temperatures and pressures. It is lighter than air and highly flammable, making it an important fuel source. It is produced naturally through the decay of organic matter by certain microorganisms, and it can also be extracted from fossil fuel deposits.
In terms of its chemical structure, methane has a tetrahedral geometry, with the carbon atom at the center and the four hydrogen atoms arranged symmetrically around it. The carbon-hydrogen bonds in methane are covalent, meaning the atoms share electrons.
Methane is considered a greenhouse gas due to its ability to absorb and trap heat from the sun, which contributes to global warming. It is emitted by various natural and human activities, such as fossil fuel combustion, livestock farming, and organic waste decomposition.
In addition to its role as a fuel and a greenhouse gas, methane has important applications in the chemical industry. It is used as a feedstock for the production of other chemicals, such as methanol, acetic acid, and hydrogen gas. Methane is also used as a precursor for the synthesis of various organic compounds and as a refrigerant in certain industrial processes.
Overall, methane plays a significant role in energy production, climate change, and the chemical industry. Its simple molecular structure and abundance make it an important area of study in the field of chemistry.
Structure and Properties of Methane
Methane is a simple hydrocarbon compound and the primary component of natural gas. It consists of one carbon atom bonded to four hydrogen atoms, with the molecular formula CH4.
Structure:
In methane, the carbon atom is located at the center of a tetrahedral structure. The four hydrogen atoms are symmetrically arranged around the carbon atom, with bond angles of approximately 109.5 degrees. The carbon-hydrogen (C-H) bonds in methane are covalent, meaning the electrons are shared between the atoms.
Properties:
1. Physical properties:
– Methane is a colorless and odorless gas at room temperature and standard pressure.
– It has a boiling point of -161.5 degrees Celsius (-258.7 degrees Fahrenheit) and a melting point of -182.5 degrees Celsius (-296.5 degrees Fahrenheit).
– Methane is lighter than air, having a density of approximately 0.717 kg/m³.
2. Chemical properties:
– Methane is highly flammable, easily igniting in the presence of oxygen and a spark or flame. When burned, it releases energy and produces carbon dioxide and water as byproducts.
– It is unreactive under normal conditions, as the carbon-hydrogen bonds in methane are relatively strong. However, methane can undergo combustion, oxidation, and certain substitution reactions when exposed to appropriate conditions or reactants.
– Methane is insoluble in water but can dissolve in nonpolar solvents like benzene or hexane.
3. Greenhouse gas:
– Methane is a potent greenhouse gas, meaning it traps heat in the Earth’s atmosphere and contributes to global warming. It has a higher global warming potential than carbon dioxide, although its atmospheric concentration is much lower.
– Sources of methane emissions include natural processes (e.g., wetlands, termites), human activities (e.g., fossil fuel extraction and combustion, agriculture, landfill waste decomposition), and industrial processes.
Overall, methane’s simple molecular structure, low reactivity, flammability, and greenhouse gas properties have significant implications in various fields, such as energy production, climate change mitigation, and environmental studies.
Occurrence and Sources of Methane
Methane (CH4) is a colorless and odorless greenhouse gas that is naturally occurring in the Earth’s atmosphere. It is the primary component of natural gas and is widely used as a fuel.
There are both natural and human-made sources of methane. Natural sources include:
1. Wetlands: Methane is produced in wetland areas such as marshes, swamps, and peatlands due to the decomposition of organic matter in oxygen-depleted environments.
2. Termites: These insects produce methane as a byproduct of their digestive processes. Termite mounds can be significant sources of methane emissions in some regions.
3. Oceans: Methane can be released from the seafloor through natural processes like microbial activity or geological activities like submarine volcanic eruptions.
4. Methanogenic bacteria: These microbes live in environments such as the intestines of ruminant animals (like cows) and produce methane during digestion.
Human activities also contribute to methane emissions. Some primary sources of anthropogenic (human-made) methane include:
1. Energy production and use: Extraction, processing, and distribution of fossil fuels, including natural gas, can result in methane leaks. Incomplete combustion of fossil fuels also leads to the release of methane.
2. Agriculture: Livestock farming, particularly of ruminant animals such as cattle, sheep, and goats, produces significant amounts of methane through their digestive processes.
3. Landfills: Methane is generated when organic waste decomposes in landfills. Proper waste management and capturing methane from landfills can help reduce emissions.
4. Rice cultivation: In flooded rice fields, bacteria decompose organic matter under anaerobic conditions, leading to the production of methane.
Reducing methane emissions is crucial because it is a potent greenhouse gas, having a much higher global warming potential than carbon dioxide over shorter time frames. Efforts to mitigate methane emissions include improving waste management, adopting more efficient livestock farming practices, and utilizing technology to prevent leaks in the production and distribution of fossil fuels.
Uses and Applications of Methane
Methane, also known as CH4, is a highly important compound in chemistry with various uses and applications. Here are some of them:
1. Fuel: Methane is the primary component of natural gas and is used extensively as a fuel source for heating, cooking, electricity generation, and transportation. It is a cleaner-burning fuel compared to other fossil fuels and is a widely used alternative to more polluting fuels.
2. Chemical Feedstock: Methane is used as a feedstock for the production of various chemicals and compounds. For example, it is a precursor for the production of methanol, formaldehyde, acetic acid, and other important chemicals. These chemicals are used for the production of plastics, solvents, pharmaceuticals, and many other products.
3. Hydrogen Production: When methane is subjected to steam reforming or partial oxidation, it can be used to produce hydrogen gas. Hydrogen is a versatile and clean-burning fuel used in fuel cells, ammonia synthesis, and various industrial processes.
4. Laboratory Applications: Methane is commonly used in laboratories as a calibration gas for gas chromatography and mass spectrometry. It is also used for leak detection due to its low ignition energy and distinct odor.
5. Environmental Applications: Methane plays a significant role in the environment as a greenhouse gas. Its study and monitoring help understand climate change and global warming. Techniques such as isotopic analysis of methane provide information about its sources, such as natural gas leaks or microbial activity in wetlands.
These are just a few examples of the many uses and applications of methane in chemistry. Its importance in fuel, chemical synthesis, laboratory analysis, and environmental studies make it a versatile compound with diverse applications.
Environmental Impact of Methane
Methane (CH4) is a greenhouse gas that plays a significant role in climate change. Its environmental impact is primarily associated with its contribution to global warming. Here are some key points regarding the environmental impact of methane in chemistry:
1. Greenhouse gas: Methane is considered a potent greenhouse gas, with a global warming potential (GWP) 28-36 times greater than carbon dioxide (CO2) over a 100-year period. Although methane concentrations in the atmosphere are much lower than CO2, its heat-trapping ability is more potent, making it an important contributor to global warming.
2. Climate change: Methane emissions have been increasing over the past few decades due to human activities such as fossil fuel extraction, livestock farming, waste disposal, and rice cultivation. The release of methane into the atmosphere contributes to the greenhouse effect, leading to global warming and climate change.
3. Carbon cycle disruption: Methane is also involved in the carbon cycle, which is a natural process of exchanging carbon between the atmosphere, land, and oceans. Large amounts of methane released from activities such as fracking or natural gas leaks can disrupt the carbon cycle, altering the balance between carbon sources and sinks.
4. Ozone depletion: Methane indirectly contributes to ozone depletion in the upper atmosphere. When methane reaches the stratosphere, it undergoes a series of chemical reactions that generate water vapor and other compounds that destroy ozone molecules. Ozone depletion leads to increased ultraviolet (UV) radiation reaching the Earth’s surface, which has harmful effects on human health and the environment.
5. Feedback loop: Methane is involved in positive feedback loops, where its release can trigger additional greenhouse gas emissions. For example, as global temperatures rise, permafrost (frozen soil) begins to thaw, releasing trapped organic matter. This organic matter decomposes, producing methane, which further contributes to global warming, triggering more permafrost thawing and methane release.
6. Air quality: Methane is not only relevant to climate change but also impacts air quality. It is a key component in the formation of ground-level ozone, a primary air pollutant that affects human health and contributes to smog formation. Additionally, methane emissions alongside certain volatile organic compounds (VOCs) can react to form secondary organic aerosols, which have adverse effects on both air quality and human respiratory health.
Reducing methane emissions is crucial for mitigating climate change and improving air quality. Strategies include capturing methane emissions from waste management systems, adopting low-emission agricultural practices, minimizing methane leaks during energy production, and promoting methane recovery and utilization technologies.

I am a passionate Chemistry teacher committed to dedicating my life to help students explore the beauty and significance of chemistry. With over 10 years of teaching experience, I focus on imparting knowledge while encouraging curiosity and deep understanding of the subject.