Introduction
Chemistry is the scientific discipline that studies the composition, properties, and behavior of matter, as well as the changes it undergoes during chemical reactions. It is a central science that bridges the gap between physics and biology, providing insights into the structure and interactions of molecules, atoms, and subatomic particles. Chemistry plays a crucial role in many aspects of our daily lives, from the development of new medicines and materials to the understanding of environmental processes and the production of energy. By investigating and manipulating the fundamental building blocks of matter, chemists can create new compounds, understand their properties, and develop strategies to solve complex problems in various fields.
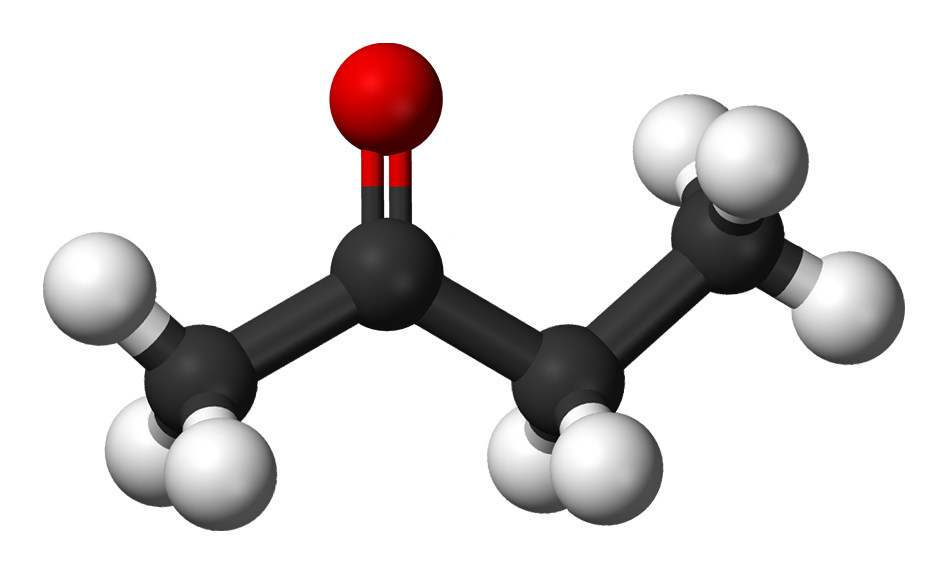
Properties of Methyl Ethyl Ketone
Synthesis of Methyl Ethyl Ketone
Methyl ethyl ketone (MEK) is synthesized through a process called acetone condensation, which involves the condensation of acetone (propanone) to form MEK.
The synthesis of MEK starts with the conversion of propanone to acetone cyanohydrin through a cyanohydrin reaction. This reaction is typically carried out by adding hydrogen cyanide (HCN) to propanone in the presence of a base, such as sodium or potassium hydroxide. The base facilitates the addition of HCN to propanone, resulting in the formation of the desired acetone cyanohydrin.
The next step involves the hydrolysis of acetone cyanohydrin to produce MEK. This process is achieved by treating the acetone cyanohydrin with an acid, such as sulfuric acid or hydrochloric acid. The acid catalyzes the hydrolysis reaction, breaking the cyanohydrin bond and producing MEK as the main product.
After the hydrolysis step, MEK can be purified through distillation or other separation techniques to obtain the desired product. Distillation allows for the separation of MEK from any remaining impurities in the reaction mixture.
Overall, the synthesis of MEK involves the conversion of propanone to acetone cyanohydrin, followed by the hydrolysis of the cyanohydrin to yield the final product, methyl ethyl ketone.
Uses of Methyl Ethyl Ketone
Environmental and Health Considerations
In chemistry, there are numerous environmental and health considerations that need to be taken into account. These considerations are essential in order to minimize the negative impacts of chemical processes on both the environment and human health. Some of the key areas of concern include:
1. Green Chemistry: Green chemistry involves the design and development of chemical processes and products that reduce or eliminate the use and generation of hazardous substances. By focusing on the principles of prevention, atom economy, and safer solvents and reactions, green chemistry aims to minimize waste, energy consumption, and environmental harm.
2. Air and Water Pollution: Many chemical processes can release pollutants into the air and water, leading to environmental contamination. These pollutants can have adverse effects on ecosystems, air quality, and water supplies. It is crucial to implement appropriate pollution control measures, such as scrubbers or filtration systems, to remove or reduce the emissions of harmful substances.
3. Toxicity and Exposure Risks: Chemical substances used in various applications can pose health risks to workers and the general population. It is important to identify and assess the toxicity of chemicals to determine safe handling procedures and exposure limits. Additionally, protective measures, such as personal protective equipment (PPE), ventilation systems, and proper containment, should be implemented to minimize exposure.
4. Waste Management: Chemical processes often generate waste products that need to be managed properly to prevent contamination of the environment. Waste should be treated, stored, and disposed of according to regulatory guidelines to prevent pollution and minimize risks to human health.
5. Energy Efficiency: Chemical processes can be energy-intensive, leading to increased carbon emissions and environmental impact. It is imperative to improve energy efficiency through process optimization, the use of alternative energy sources, and the development of more sustainable reaction pathways.
6. Life Cycle Assessment: Evaluating the environmental impacts of chemical processes requires considering the entire life cycle, from raw material extraction to product disposal. Life cycle assessment (LCA) helps identify potential environmental hotspots and supports the development of more sustainable and resource-efficient processes.
Overall, considering environmental and health implications in chemistry is crucial for promoting sustainable practices, reducing pollution, protecting ecosystems, and safeguarding human health. Embracing these considerations can lead to the development of greener and safer chemical processes and products.

Abigail Gutmann Doyle is a renowned Organic chemistry professor in Los Angeles. Her research focuses on the development of new chemical transformations in organic chemistry. She has won awards such as: Bayer Early Excellence in Science Award, Phi Lambda Upsilon National Fresenius Award, Presidential Early Career Award for Scientists and Engineers, BMS Unrestricted Grant in Synthetic Organic Chemistry.