Introduction to Phenol (C₆H₅OH)
Chemistry is the branch of science that deals with the composition, structure, properties, and transformation of matter. It is considered the central science as it connects and intersects with other scientific disciplines such as physics, biology, and materials science.
In chemistry, scientists study the interactions between atoms, molecules, and ions, seeking to understand how they combine and interact to form new substances. This involves investigating various chemical reactions, such as combustion, oxidation, and synthesis, to uncover the underlying principles and mechanisms that govern these processes.
Chemistry is divided into several sub-disciplines, including organic chemistry, inorganic chemistry, physical chemistry, analytical chemistry, and biochemistry. Each of these areas focuses on specific aspects of matter and its properties.
Organic chemistry focuses on the study of carbon compounds, the building blocks of life. It involves understanding the chemical structures, reactions, and properties of organic molecules, such as hydrocarbons, carbohydrates, and proteins.
Inorganic chemistry, on the other hand, deals with the study of non-carbon compounds, including metals, minerals, and non-metallic elements. It investigates the properties, structures, and reactions of these compounds, which are widely used in various industries and applications.
Physical chemistry combines principles of physics and chemistry to study the behavior of matter and energy at the molecular and atomic level. It involves understanding concepts such as thermodynamics, quantum mechanics, and spectroscopy to explain the properties and transformations of matter.
Analytical chemistry focuses on the development and use of techniques and instruments to analyze and measure the composition and properties of substances. It plays a vital role in fields such as environmental monitoring, forensic science, and pharmaceutical analysis.
Finally, biochemistry explores the chemical processes and substances that occur within living organisms. It investigates the structure and function of biological molecules, such as proteins, nucleic acids, and enzymes, and their roles in various biological processes.
Chemistry has countless practical applications, ranging from the development of new medicines and materials to the understanding of environmental processes and the production of energy. With its emphasis on critical thinking, problem-solving, and laboratory skills, chemistry provides a foundation for many other scientific disciplines and industries.
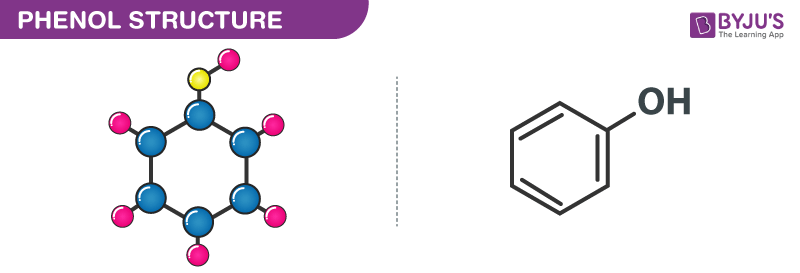
Chemical Structure of Phenol (C₆H₅OH)
The chemical structure of phenol (C₆H₅OH) consists of a benzene ring with a hydroxyl (-OH) group attached to one of the carbon atoms in the ring. Here is the structural formula for phenol:
H
|
H - C - C - C - C - C - C - H
|
OH
In this representation:
- The hexagonal structure represents the benzene ring, which consists of six carbon atoms (labeled as C) and six hydrogen atoms (labeled as H).
- The -OH group is attached to one of the carbon atoms in the benzene ring, replacing one of the hydrogen atoms. This hydroxyl group gives phenol its characteristic chemical properties and makes it an aromatic alcohol.
Properties of Phenol
Phenol (C₆H₅OH) exhibits various physical and chemical properties that are essential to its applications and reactivity. Here are some key properties of phenol:
Physical State: Phenol can exist as a white crystalline solid at room temperature, although it can also be a colorless liquid. Its physical state depends on temperature and impurities. The solid form is a relatively high-melting compound, with a melting point of about 40-41°C.
Odor: Phenol has a characteristic sweet, tarry, and medicinal odor, which is quite distinct and easily recognizable. This odor can be somewhat unpleasant in higher concentrations.
Solubility: Phenol is moderately soluble in water. It forms a weakly acidic aqueous solution due to the presence of the hydroxyl group, which can release protons (H⁺ ions). However, it is much more soluble in organic solvents like ether, chloroform, and ethanol.
Melting and Boiling Points: Phenol melts at around 40-41°C and boils at approximately 182°C. These physical properties can vary slightly depending on the purity of the substance.
Acidity: Phenol is a weak acid. The hydroxyl group attached to the aromatic ring can release a proton (H⁺ ion), making phenol slightly acidic. It can undergo reactions with strong bases to form phenolate ions.
Aromaticity: Phenol is an aromatic compound because it contains a benzene ring in its structure. The electrons in the benzene ring are delocalized, giving the compound its characteristic stability and reactivity.
Toxicity: Phenol is toxic and can be harmful to humans. Exposure to phenol can cause skin and eye irritation, respiratory problems, and other health issues. Proper safety precautions, such as wearing protective clothing and handling it in well-ventilated areas, are necessary when working with this compound.
Reactivity: Phenol can participate in various chemical reactions, including electrophilic aromatic substitution reactions and nucleophilic substitution reactions at the hydroxyl group. It can undergo processes like halogenation, nitration, and sulfonation.
Antiseptic Properties: Historically, phenol has been used as an antiseptic and disinfectant due to its ability to kill microorganisms. However, it has been largely replaced by safer alternatives for medical and personal care purposes.
Industrial Applications: Phenol is a crucial raw material in the production of a wide range of chemicals and plastics, making it an important compound in the chemical industry.
Environmental Impact: The release of phenol into the environment, if not properly managed, can be harmful to aquatic life and ecosystems. Regulations are in place to control its discharge into water bodies.
These properties of phenol make it a versatile and important chemical compound, but it should be handled with care due to its toxicity and potentially harmful effects on human health and the environment.
Uses of Phenol
Phenol (C₆H₅OH) is a versatile chemical compound with a wide range of uses in various industries and applications. Some of its key uses include:
- Chemical Intermediates: Phenol serves as a valuable intermediate in the production of many important chemicals and compounds. It is used in the synthesis of pharmaceuticals, dyes, and perfumes.
- Plastics and Resins: Phenol is a fundamental raw material for the production of phenolic resins and plastics, such as Bakelite. These materials find applications in manufacturing electrical insulators, laminates, coatings, and adhesives.
- Pharmaceuticals: Phenol is employed in the production of various pharmaceuticals, including analgesics, antiseptics, and disinfectants. While it was historically used as an antiseptic, it has been largely replaced by safer alternatives.
- Agrochemicals: Phenol can be used in the formulation of herbicides, fungicides, and pesticides, helping protect crops from pests and diseases.
- Flavor and Fragrance: In lower concentrations, phenol is used in the food and fragrance industries to impart smoky or medicinal flavors and scents to products like smoked meats, spices, and perfumes.
- Laboratory Reagents: Phenol is used as a reagent in chemical laboratories for a variety of chemical reactions and syntheses. It is often used as a mild reducing agent and in some DNA extraction procedures.
- Resorcinol Production: Phenol is a precursor in the synthesis of resorcinol, which is used in the production of adhesives, flame retardants, and skin-lightening products.
- Coal Tar and Creosote: Phenol is a component of coal tar, and it can be extracted from coal tar along with other useful chemicals. Creosote, a wood preservative, is also derived from phenol.
- Epoxy Resins: Phenol is used in the production of epoxy resins, which find applications in coatings, adhesives, and composites for construction and industrial uses.
- Textile Industry: Phenol is employed in the dyeing and printing of textiles and helps fix dyes to fabric fibers.
- Paper Industry: It is used in the production of resins for adhesives used in the paper and packaging industries.
- Oil and Gas: In the oil and gas sector, phenol can be used for inhibiting the formation of hydrates and corrosion in pipelines and equipment.
- Extraction Solvent: Phenol is used as a solvent for the extraction of certain organic compounds, including resins, oils, and alkaloids.
- Phenol-formaldehyde Resins: These resins, derived from phenol, are utilized in the production of molded products, including buttons, kitchenware, and automotive parts.
- Adhesives and Sealants: Phenol-based adhesives are used in various applications, such as bonding plywood and making laminated wood products.
It’s important to note that the use of phenol, particularly in applications involving human contact, has decreased over time due to its toxicity and the availability of safer alternatives. Strict safety measures and regulations are in place to mitigate the risks associated with its handling and disposal.
Safety and Precautions
Handling phenol (C₆H₅OH) requires careful precautions due to its toxicity and potential health hazards. Here are some important safety measures and precautions when working with phenol:
Personal Protective Equipment (PPE):
Wear appropriate PPE, including lab coats, chemical-resistant gloves, safety goggles, and, if necessary, a face shield. Skin contact with phenol should be minimized.
Ventilation:
Use phenol in a well-ventilated area, such as a fume hood, to prevent the inhalation of vapors. Adequate ventilation helps disperse and reduce the concentration of phenol in the air.
Respiratory Protection:
Depending on the concentration and form of phenol being handled, respiratory protection, such as a chemical cartridge respirator, may be required to protect against inhalation of vapors.
Avoid Ingestion:
Do not eat, drink, or smoke while working with phenol. Wash hands thoroughly after handling the compound to prevent accidental ingestion.
Chemical Spill Response:
Be prepared to respond to chemical spills. In case of a spill, follow appropriate spill control procedures and use absorbent materials to contain and clean up the spill.
Eye and Skin Contact:
In case of skin contact, immediately remove contaminated clothing and flush the affected area with copious amounts of water. If phenol contacts the eyes, rinse them thoroughly with water for at least 15 minutes and seek medical attention.
First Aid:
Familiarize yourself with first-aid measures for phenol exposure. Have an eyewash station and an emergency shower available in the vicinity.
Storage:
Store phenol in a cool, dry, well-ventilated area away from incompatible materials, such as strong oxidizing agents. Keep containers tightly sealed and clearly labeled.
Handling and Transfer:
Use chemical-resistant glass or plastic containers for handling and transferring phenol. Avoid using reactive materials like metals for handling.
Compatibility:
Be aware of the reactivity of phenol with other chemicals. It can react with strong bases, oxidizing agents, and some metals, leading to potentially hazardous situations.
Waste Disposal:
Dispose of phenol waste in accordance with local, state, and federal regulations. Phenol waste should be collected and treated as hazardous waste.
Emergency Procedures:
Know the location of emergency equipment, such as eye wash stations, emergency showers, and fire extinguishers. Familiarize yourself with emergency procedures in case of accidental exposure or fires.
Training:
Ensure that personnel handling phenol are adequately trained in the safe use, handling, and disposal of the chemical.
Regulatory Compliance:
Comply with all relevant safety regulations, guidelines, and legal requirements related to the handling of phenol, which may vary by region.
Safety Data Sheet (SDS):
Always refer to the Safety Data Sheet (SDS) provided by the manufacturer for specific safety information, handling instructions, and emergency response guidance for the phenol product in use.
Phenol should be handled with great care, and those working with it should be familiar with its properties and potential hazards. Following the recommended safety precautions and practices is essential to mitigate the risks associated with phenol exposure.

Abigail Gutmann Doyle is a renowned Organic chemistry professor in Los Angeles. Her research focuses on the development of new chemical transformations in organic chemistry. She has won awards such as: Bayer Early Excellence in Science Award, Phi Lambda Upsilon National Fresenius Award, Presidential Early Career Award for Scientists and Engineers, BMS Unrestricted Grant in Synthetic Organic Chemistry.