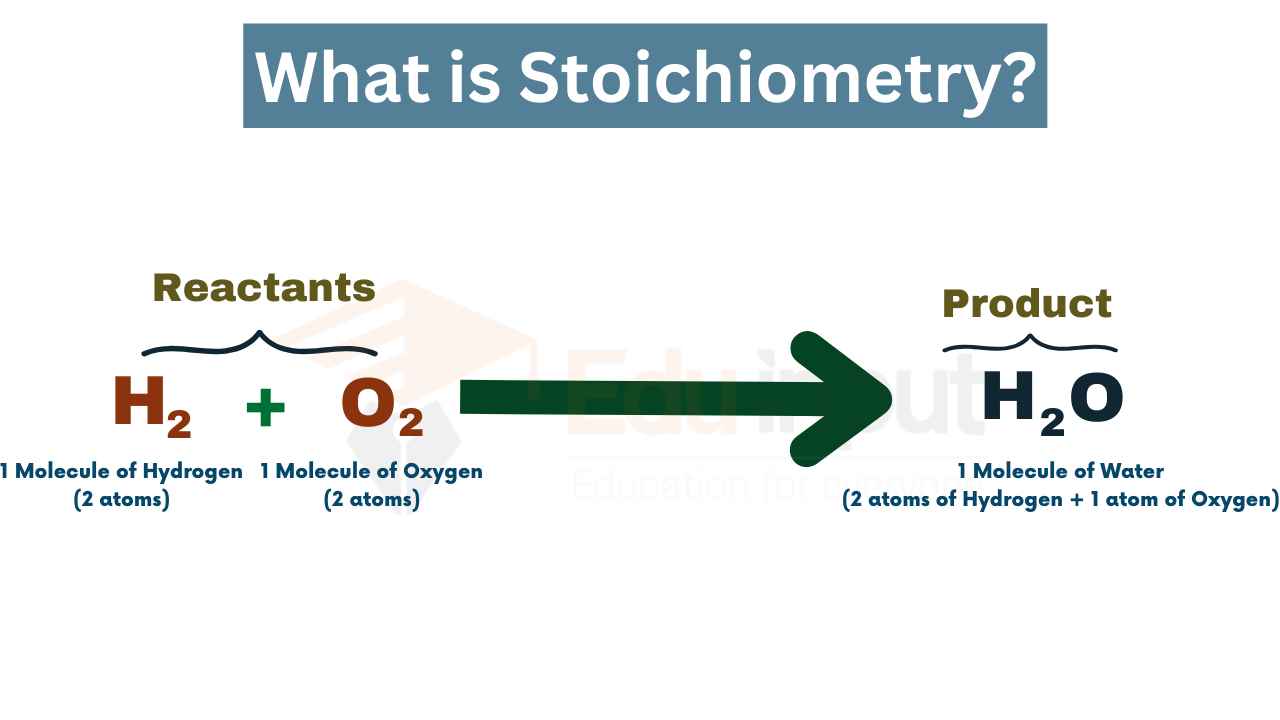
Definition of Stoichiometry
Stoichiometry is a branch of chemistry that deals with quantitative relationships between the amounts of reactants and products in chemical reactions. It involves the analysis of the ratios and proportions of the substances involved in a chemical reaction, as well as the calculation of the amounts of reactants required and the amounts of products produced. By applying stoichiometric principles, chemists can determine the theoretical yield of a reaction, predict the amount of product obtained from a given amount of reactants, and determine the limiting reactant. Stoichiometry is essential for understanding and predicting the outcomes of chemical reactions and is widely used in various areas of chemistry, including synthesis, analysis, and industrial processes.
Importance of Stoichiometry in Chemistry
Stoichiometry is a fundamental concept in chemistry that plays a crucial role in understanding and predicting the outcome of chemical reactions. It helps determine the quantitative relationship between reactants and products in a balanced chemical equation. Here are some key reasons why stoichiometry is important in chemistry:
1. Conservation of Mass: Stoichiometry is based on the fundamental principle of conservation of mass, which states that matter cannot be created or destroyed during a chemical reaction. By using stoichiometry, we can balance chemical equations and ensure that the total mass of the reactants equals the total mass of the products.
2. Reaction Prediction: Stoichiometry allows us to predict the possible products of a chemical reaction based on the given amounts of reactants. With the help of stoichiometric calculations, chemists can determine the limiting reactant, which is the reactant that is completely consumed and determines the maximum amount of product that can be formed.
3. Quantitative Analysis: Stoichiometry facilitates quantitative analysis in chemistry. By knowing the stoichiometric ratios between reactants and products, we can calculate the amounts (in moles, mass, or volume) of substances involved in a chemical reaction. This enables us to determine the concentration of solutions, the mass of a product in a reaction, or the volume of gases produced or consumed.
4. Experimental Design: Stoichiometry is essential for designing experiments in the laboratory. By using stoichiometry, chemists can calculate the exact amounts of reactants needed to obtain a desired amount of product. This helps to optimize reaction conditions, minimize waste, and ensure the efficiency of chemical processes.
5. Stoichiometric Calculations: Stoichiometry involves various types of calculations, such as mole-to-mole, mole-to-mass, and mass-to-mass conversions. These calculations allow chemists to interconvert between different units of measurement and relate the quantities of substances in a chemical equation.
6. Yield and Efficiency: Stoichiometry is important for determining the theoretical yield of a chemical reaction, which is the maximum amount of product that can be obtained according to stoichiometry. By comparing the actual yield to the theoretical yield, chemists can calculate the percent yield, which measures the efficiency of a reaction.
Overall, stoichiometry is essential in chemistry as it provides a quantitative understanding of chemical reactions, enables prediction and analysis of reaction outcomes, and supports experimental design and optimization of chemical processes.
Stoichiometric Calculations and Equations
In chemistry, stoichiometric calculations and equations are used to determine the relationships between the amounts of reactants and products in a chemical reaction. These calculations are based on the principles of stoichiometry, which is the study of the quantitative relationships between the substances involved in a chemical reaction.
Stoichiometry is based on the balanced chemical equation of a reaction, which shows the reactants on the left side and the products on the right side. The coefficients in front of the reactants and products indicate the relative number of moles of each substance involved.
Stoichiometric calculations involve using the balanced equation to determine the molar ratios between the reactants and products. This allows us to calculate the amount of one substance given the amount of another substance. These calculations can be used to determine the limiting reactant, the theoretical yield, and to plan and optimize chemical reactions.
To perform stoichiometric calculations, the following steps are typically followed:
1. Write the balanced chemical equation for the reaction.
2. Determine the molar mass of the reactants and products involved.
3. Convert the given quantities of reactants or products to moles using their molar mass.
4. Use the coefficients in the balanced equation to establish the mole ratios between the substances.
5. Calculate the desired quantity by multiplying the number of moles of the known substance by the mole ratio.
6. If necessary, convert the quantity back to the desired units.
For example, consider the reaction between hydrogen gas (H2) and oxygen gas (O2) to form water (H2O):
2H2 + O2 -> 2H2O
If we are given the mass of hydrogen gas and asked to calculate the mass of water produced, we would follow these steps:
1. Write the balanced chemical equation: 2H2 + O2 -> 2H2O
2. Determine the molar masses: H2 = 2 g/mol, O2 = 32 g/mol, H2O = 18 g/mol
3. Convert the mass of hydrogen gas to moles: Moles of H2 = given mass / molar mass = X g / 2 g/mol = X/2 mol
4. Use the mole ratio from the equation: Moles of H2O = (X/2 mol H2) * (2 mol H2O / 2 mol H2) = X mol H2O
5. Convert the moles of water to mass: Mass of H2O = moles of H2O * molar mass = X mol * 18 g/mol = 18X g H2O
By following these calculations, we can determine the amount of water produced when a certain mass of hydrogen gas reacts with oxygen gas.
Stoichiometric calculations and equations are important tools in chemistry, helping us to understand and quantify chemical reactions. They allow us to predict the outcomes of reactions, determine reaction yields, and optimize chemical processes.
Limiting Reactants and Excess Reactants
In chemistry, limiting reactants and excess reactants are terms used to describe the amounts of reactants involved in a chemical reaction.
A limiting reactant, also known as a limiting reagent, is the reactant that is completely consumed in a reaction, thereby limiting the amount of product that can be formed. It determines the maximum amount of product that can be obtained.
To determine the limiting reactant, you need to compare the ratio of moles of reactants to the stoichiometric ratio of the reaction. The reactant with a ratio that is lower than the stoichiometric ratio is the limiting reactant.
On the other hand, an excess reactant is the reactant that is present in an amount greater than necessary to react with the limiting reactant. It is not completely consumed in the reaction and is left over after the reaction is complete.
To calculate the amount of excess reactant remaining after the reaction, you can subtract the amount of the limiting reactant consumed from the initial amount of the excess reactant.
Limiting reactants and excess reactants are important concepts in chemistry because they determine the efficiency of a reaction and the amount of product that can be obtained. It is essential to identify the limiting reactant to know the theoretical yield of a reaction and to determine the appropriate amount of reactants to use in order to maximize the production of the desired product.
Applications of Stoichiometry in Real-Life Situations
1. Drug formulation: Stoichiometry is crucial in pharmaceutical chemistry to determine the optimal ratio of reactants needed to produce a specific drug. By applying stoichiometry principles, chemists can calculate the quantities of each reactant required and ensure the desired chemical reactions proceed efficiently.
2. Environmental monitoring: Stoichiometry plays a role in understanding and monitoring pollution levels in the environment. By knowing the stoichiometry of reactions involving pollutants, scientists can determine the amount of pollutants released into the air or water based on the concentration of other chemicals present.
3. Fuel combustion: Stoichiometry is essential in determining the ideal air-to-fuel ratio needed for complete combustion of hydrocarbon fuels. By balancing the chemical equation and applying stoichiometric calculations, engineers can optimize the combustion process and ensure maximum efficiency while minimizing emissions.
4. Food industry: Stoichiometry is used in food chemistry to calculate the exact quantities of reactants required for certain reactions, such as fermentation or caramelization. By applying stoichiometric calculations, food scientists can ensure consistency and quality in food production.
5. Electrochemistry: Stoichiometry is also relevant in electrochemical applications, such as in the production of batteries or electrolysis processes. By understanding the stoichiometry involved, engineers can design and optimize electrochemical systems for efficient energy storage or chemical synthesis.
6. Environmental remediation: Stoichiometry is used to determine the appropriate amounts of reactants to use in environmental remediation processes. For example, stoichiometric calculations can help determine the quantity of a specific chemical required to neutralize contaminants in soil or water.
7. Fertilizer production: Stoichiometry is essential in the manufacturing of fertilizers. By applying stoichiometric calculations, chemical engineers can determine the precise ratios of elements needed to produce fertilizers that provide the necessary nutrients for plant growth.
8. Industrial processes: Stoichiometry plays a crucial role in various industrial processes, such as the production of chemicals, plastics, and fuels. By understanding the stoichiometry of the reactions involved, engineers can optimize production processes, minimize waste, and ensure cost-effective manufacturing.
9. Air quality control: Stoichiometry is used in air quality monitoring and control, particularly in understanding the reactions involved in the formation of pollutants such as ozone or nitrogen oxides. By applying stoichiometric principles, scientists can predict the levels of these pollutants based on precursor chemicals and develop strategies to mitigate their harmful effects.
10. Water treatment: Stoichiometry is applied in water treatment processes to determine the optimal amount of chemicals needed to remove contaminants. For example, stoichiometric calculations can help determine the appropriate dosage of coagulants or disinfectants to treat water and ensure it meets regulatory standards.

I am a passionate Chemistry teacher committed to dedicating my life to help students explore the beauty and significance of chemistry. With over 10 years of teaching experience, I focus on imparting knowledge while encouraging curiosity and deep understanding of the subject.