Introduction to Sucrose
Sucrose is a type of carbohydrate commonly known as table sugar. It is a white, crystalline solid that is found naturally in many plants, including sugar cane and sugar beets. Sucrose is a disaccharide molecule, meaning that it is composed of two monosaccharide units joined together through a glycosidic bond.
Chemically, sucrose is made up of one glucose molecule bonded to one fructose molecule. This makes it a combination of a glucose unit and a fructose unit. The bond between the two units is formed through a condensation reaction, resulting in the release of a molecule of water.
Sucrose is a non-reducing sugar, meaning that it does not readily undergo oxidation or reduction reactions. This is due to the absence of any free aldehyde or ketone functional groups in its structure. The glycosidic bond between the glucose and fructose units makes sucrose a stable molecule that can withstand various environmental conditions without being easily broken down.
In addition to its sweet taste, sucrose is widely used as a sweetener in the food industry. It is also commonly used as a source of energy in the human body. When consumed, enzymes in the digestive system break down sucrose into its constituent glucose and fructose units, which can then be absorbed by the body and used as a source of energy.
Overall, sucrose is an important compound in chemistry due to its significance in food science and biochemistry. Its unique structure and properties make it a versatile substance with a wide range of applications.
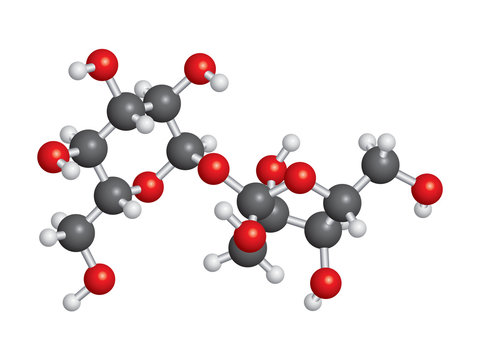
Chemical Composition of Sucrose (C₁₂H₂₂O₁₁)
Sucrose, also known as table sugar, has the chemical formula C₁₂H₂₂O₁₁. It is a disaccharide composed of glucose and fructose molecules. The chemical structure of sucrose consists of a glucose molecule bonded to a fructose molecule through a glycosidic linkage.
The breakdown of the chemical composition of sucrose is as follows:
– Carbon (C): 12 atoms
– Hydrogen (H): 22 atoms
– Oxygen (O): 11 atoms
Sucrose is a white, crystalline solid that is soluble in water. It is commonly used as a sweetener in various food and beverage products.
Structure and Molecular Formula of Sucrose
The structure of sucrose is a disaccharide made up of two monosaccharide units, glucose and fructose. Its molecular formula is C12H22O11. Sucrose consists of glucose and fructose units linked by a glycosidic bond between the carbon atoms C1 of glucose and C2 of fructose. The structure can be represented as follows:
HO-C-C(=O)-C(HOH)-C(HOH)-C(HOH)-C(HOH)-C(HOH)-C(HOH)-C(HOH)-C(HOH)-C(HOH)-C(HOH)-C(HOH)-C(HOH)-C(HOH)-C(HOH)-C(O)-C-C(HOH)-O
Each “HO” represents a hydroxyl group (-OH) and “(=O)” represents a carbonyl group.
Properties and Characteristics of Sucrose
Sucrose, also known as table sugar, is a common disaccharide composed of glucose and fructose. It has the following properties and characteristics in chemistry:
1. Molecular Formula: The molecular formula of sucrose is C12H22O11.
2. Structure: Sucrose is a non-reducing sugar and has a glycosidic bond between the glucose and fructose units. It has a symmetrical structure with a hemiacetal linkage (glucosidic bond) formed between the C1 of glucose and C2 of fructose.
3. Solubility: Sucrose is highly soluble in water, forming a clear, sweet-tasting solution. It is relatively insoluble in non-polar solvents, such as organic solvents.
4. Melting and Boiling Points: Sucrose has a melting point of approximately 185-186°C (365-367°F) and does not have a defined boiling point since it decomposes at high temperatures.
5. Sweetness: Sucrose is a naturally occurring sweetener and has a high level of sweetness. It is approximately 1.5 times sweeter than an equimolar solution of glucose or fructose.
6. Stability: Sucrose is relatively stable under normal conditions but begins to break down slowly at temperatures above its melting point. Heating sucrose above its decomposition temperature leads to the formation of caramel.
7. Reducing Properties: Sucrose itself is a non-reducing sugar, meaning it does not undergo oxidation reactions. However, sucrose can be hydrolyzed into its component monosaccharides (glucose and fructose) when treated with acid or enzymes.
8. Optical Activity: Sucrose is optically active due to the presence of chiral carbons in its structure. It rotates plane-polarized light to the right, with a specific rotation of +66.5° in a 1g/100mL solution at a wavelength of 589 nm.
9. Food Uses: Sucrose is widely used as a sweetener in various food and beverage products due to its pleasant taste. It provides energy in the form of carbohydrates and is commonly used in baking, confectionery, and soft drinks.
10. Preservation: Sucrose has been used traditionally as a preservative for fruits and other food items. It can help to inhibit microbial growth and extend the shelf life of certain products.
These properties and characteristics make sucrose a versatile compound with various applications in food science, biochemistry, and other fields of chemistry.
Uses and Importance of Sucrose
Sucrose, also known as table sugar, is an important compound in chemistry with various uses and importance. Here are a few:
1. Solvent: Sucrose is soluble in water, making it a useful solvent in various chemical reactions and processes. It can dissolve other compounds, allowing for their interaction and chemical reactions to take place.
2. Energy source: Sucrose is a carbohydrate that can be metabolized in the body to produce energy. Its molecular structure provides a readily available source of energy, making it important for cellular processes and bodily functions.
3. Food and beverage industry: Sucrose is widely used as a sweetening agent in the food and beverage industry. It enhances the taste of various food products and acts as a preservative in some cases. It is a key ingredient in baking, confectionery, soft drinks, and desserts.
4. Biomass feedstock: Sucrose can be derived from plant materials rich in carbohydrates, such as sugarcane and sugar beet. These plants serve as important biomass feedstocks for the production of sucrose, which can be further converted into other valuable chemicals and biofuels.
5. Chemical reactions: Sucrose can undergo various chemical reactions due to its functional groups. For example, it can be hydrolyzed to form glucose and fructose when treated with acid or enzymes. This hydrolysis reaction is essential in the production of invert sugar, which is widely used in the food industry.
6. Laboratory applications: Sucrose is often used in laboratory experiments and procedures. It can be used as a calibration standard in instruments like polarimeters. Sucrose solutions are also commonly used for density gradient centrifugation, a technique used in biochemistry and molecular biology to separate molecules based on their density.
Overall, the uses and importance of sucrose in chemistry are vast, ranging from its application as a sweetener and energy source to its use as a solvent and chemical reactant. Its availability and versatile nature make it a fundamental compound in various industries and laboratory settings.

Abigail Gutmann Doyle is a renowned Organic chemistry professor in Los Angeles. Her research focuses on the development of new chemical transformations in organic chemistry. She has won awards such as: Bayer Early Excellence in Science Award, Phi Lambda Upsilon National Fresenius Award, Presidential Early Career Award for Scientists and Engineers, BMS Unrestricted Grant in Synthetic Organic Chemistry.