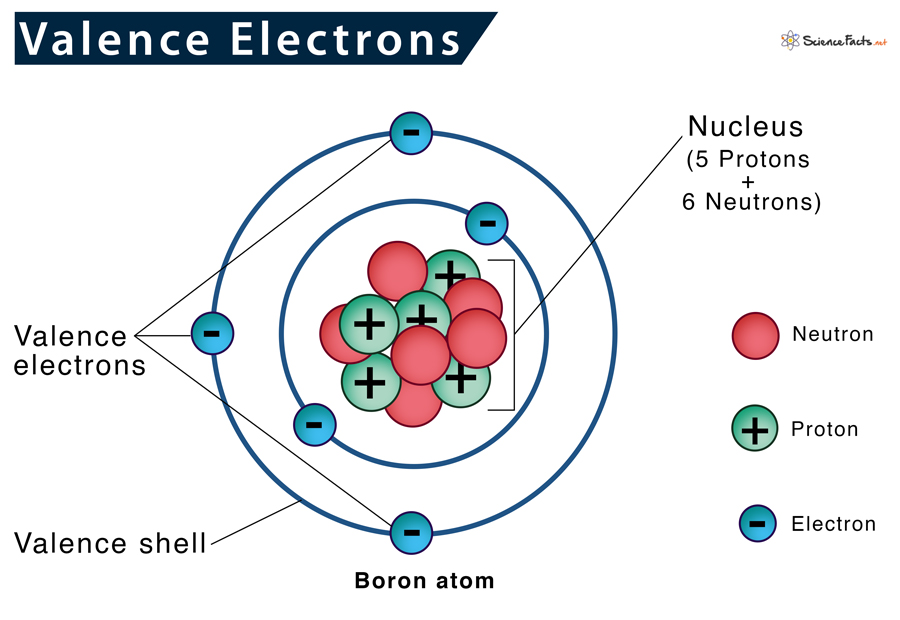
Definition of Valence Electron
In chemistry, a valence electron refers to an electron that is located in the outermost energy level or shell of an atom. These electrons are involved in chemical reactions and bonding between atoms. The number of valence electrons in an atom determines its chemical behavior, such as its reactivity and the types of compounds it can form. Valence electrons can be represented using Lewis dot structures or electron dot diagrams to illustrate the arrangement of electrons around an atom’s symbol. The concept of valence electrons is important in understanding the formation of chemical bonds and the properties of elements.
Importance of Valence Electrons in Chemistry
Valence electrons play a crucial role in chemistry as they are the outermost electrons in an atom. These electrons are involved in the formation of chemical bonds, which determine the reactivity and stability of elements and compounds.
1. Chemical bonding: Valence electrons are responsible for the formation of chemical bonds between atoms. They can be shared, transferred, or reorganized to achieve a more stable electron configuration. The number of valence electrons affects an atom’s ability to form bonds. Atoms with fewer valence electrons tend to lose or share electrons to achieve a full outer shell, while those with more valence electrons tend to gain or share electrons to achieve a full outer shell.
2. Reactivity: The number of valence electrons determines an atom’s reactivity. Elements with a low number of valence electrons tend to be more reactive as they readily lose or share electrons to achieve a stable electron configuration. Conversely, elements with a high number of valence electrons tend to be less reactive as they have a strong tendency to gain or share electrons to achieve a stable configuration.
3. Chemical properties: Valence electrons determine an element’s chemical properties. Elements with the same number of valence electrons tend to have similar chemical behaviors and properties. For example, all elements in Group 1 of the periodic table (alkali metals) have one valence electron and share similar characteristics such as high reactivity and the tendency to lose that single electron to achieve stability.
4. Ion formation: Valence electrons are involved in the formation of ions. When an atom gains or loses electrons, it becomes charged and is called an ion. The number of valence electrons determines the charge an ion will have. For example, atoms of Group 2 elements tend to lose two valence electrons to form positively charged ions with a +2 charge.
5. Molecular geometry: Valence electrons also determine the molecular geometry of compounds. Electron pairs in the valence shell repel each other, leading to specific molecular shapes. The arrangement of valence electrons influences the bond angles and overall shape of molecules. Understanding the valence electrons allows chemists to predict the molecular shape and properties of compounds.
In summary, valence electrons are crucial in determining the chemical properties, reactivity, bonding, ion formation, and molecular geometry of elements and compounds. A thorough understanding of valence electrons is fundamental for studying and manipulating chemical reactions.
Role of Valence Electrons in Chemical Bonding
Valence electrons play a crucial role in chemical bonding. Valence electrons are the outermost electrons in an atom, located in the highest energy level or valence shell. These electrons are involved in the formation of chemical bonds between atoms.
Valence electrons determine an atom’s reactivity and its ability to participate in bonding because they are the electrons that are most easily lost, gained, or shared. The number of valence electrons influences an atom’s chemical properties and determines its position in the periodic table. Elements in the same group or column on the periodic table have the same number of valence electrons, which leads to similar chemical behavior.
Chemical bonding occurs when atoms interact with each other to form compounds. Valence electrons are either shared or transferred between atoms to achieve a complete outer electron shell and attain a stable electron configuration, following the octet rule. This rule states that atoms tend to gain, lose, or share electrons to acquire a full set of eight electrons in their valence shell (except for hydrogen and helium, which only require two electrons to satisfy their valence shells).
In ionic bonding, valence electrons are transferred from one atom to another, resulting in the formation of positively charged cations and negatively charged anions. The resulting attractive electrostatic forces between the oppositely charged ions hold the compound together.
In covalent bonding, valence electrons are shared between atoms. This sharing allows atoms to achieve electron configurations similar to noble gases, which have a full valence shell. By sharing electrons, atoms can form a stable and mutually beneficial arrangement.
In metallic bonding, valence electrons are delocalized and form a sea of electrons that surround positively charged metal ions. This bond type gives rise to unique properties in metals such as high electrical conductivity and malleability.
In summary, valence electrons are crucial for chemical bonding as they determine the type and strength of bonds that can form between atoms. They govern the reactivity and chemical behavior of elements, allowing them to form compounds and achieve more stable electron configurations.
Valence Electrons and the Periodic Table
In chemistry, valence electrons refer to the electrons in the outermost energy level, or shell, of an atom. These electrons are responsible for the chemical properties and reactivity of the atom.
The periodic table is a way to organize and display the elements based on their atomic number and chemical properties. It is divided into periods, which represent the number of electron shells, and groups, which indicate the number of valence electrons.
The groups in the periodic table are numbered from 1 to 18, and each group corresponds to a specific number of valence electrons. For example, elements in Group 1 (alkali metals) have one valence electron, while elements in Group 2 (alkaline earth metals) have two valence electrons. The transition metals, located in the middle of the periodic table, have varying numbers of valence electrons.
By understanding the group number of an element on the periodic table, we can easily determine the number of valence electrons it has. This information helps us understand the element’s chemical properties, such as its bonding behavior and tendency to gain or lose electrons. Valence electrons are crucial in predicting the reactivity and behavior of elements in chemical reactions.
Examples and Applications of Valence Electrons in Chemical Reactions
Valence electrons play a crucial role in chemical reactions, as they are the outermost electrons of an atom that are involved in bond formation and the redistribution of electrons during a reaction. Here are some examples and applications of valence electrons in chemical reactions:
1. Bond formation: Valence electrons are responsible for forming chemical bonds between atoms. For example, in the reaction between hydrogen and oxygen to form water (H2O), the valence electrons of hydrogen and oxygen are used to form covalent bonds.
2. Redox reactions: Valence electrons are involved in the transfer of electrons during oxidation-reduction (redox) reactions. In these reactions, one species loses electrons (is oxidized) while another gains electrons (is reduced). Valence electrons are the ones that participate in this electron transfer. For example, during the reaction between magnesium metal and oxygen to form magnesium oxide, magnesium loses two valence electrons to oxygen.
3. Lewis dot structures: Valence electrons are used to draw Lewis dot structures, which are diagrams that represent the valence electrons as dots around the atomic symbol. These structures provide a visual representation of how valence electrons are shared or transferred during chemical reactions. They are useful in predicting the likelihood of bond formation and the overall shape of molecules.
4. Ion formation: Valence electrons can be either gained or lost by an atom, resulting in the formation of ions. By gaining or losing electrons, atoms can achieve a more stable electron configuration. The number of valence electrons determines the charge of the formed ion. For example, sodium (Na) has one valence electron, which it loses to form a sodium ion (Na+). Chlorine (Cl) has seven valence electrons, and it gains one electron to form a chloride ion (Cl^-).
5. Chemical reactivity: The number and arrangement of valence electrons greatly influence the chemical reactivity of an atom. Elements with a full valence shell of electrons (e.g., noble gases) are generally chemically inert because they do not readily gain or lose electrons. Conversely, elements with incomplete valence shells tend to be more reactive as they try to achieve a stable electron configuration by gaining, losing, or sharing electrons.
In summary, valence electrons are crucial in chemical reactions and have numerous applications in understanding and predicting the behavior of atoms and molecules. They determine the formation of chemical bonds, electron transfer in redox reactions, the drawing of Lewis dot structures, the formation of ions, and the overall reactivity of an atom.

I am a passionate Chemistry teacher committed to dedicating my life to help students explore the beauty and significance of chemistry. With over 10 years of teaching experience, I focus on imparting knowledge while encouraging curiosity and deep understanding of the subject.