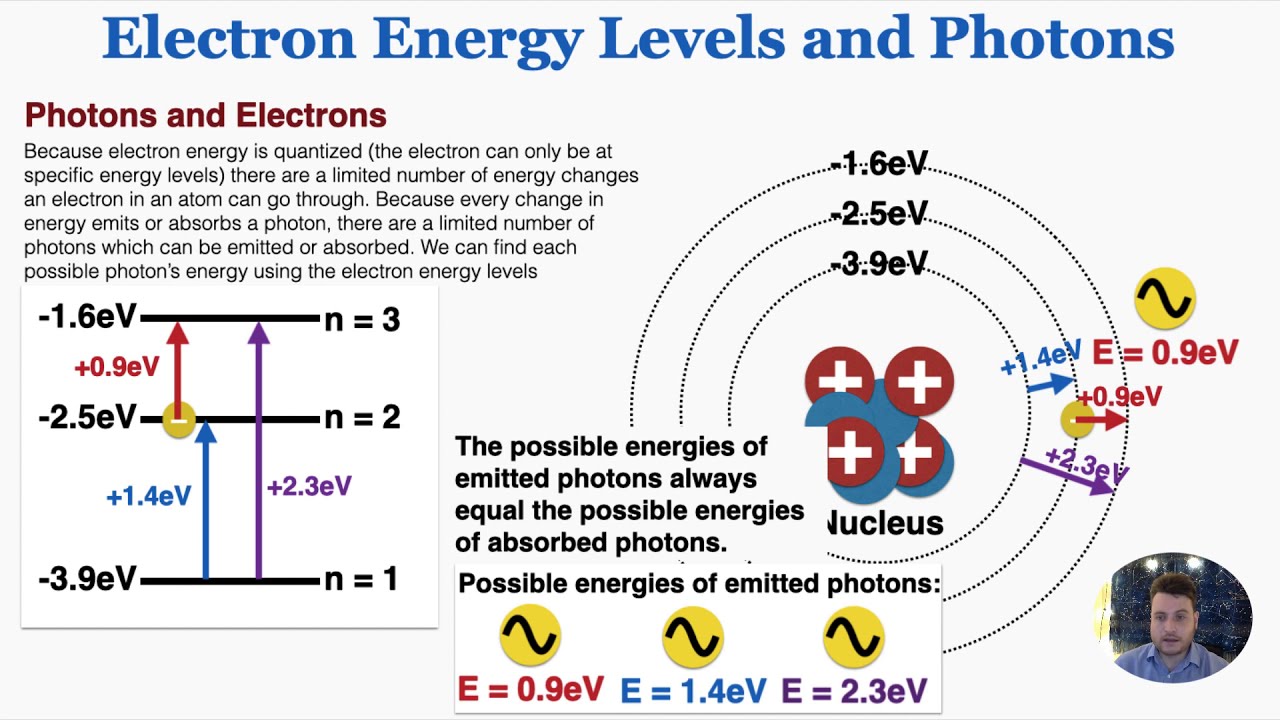
Introduction
Introduction:
Photon emission refers to the process in which photons, which are packets of electromagnetic radiation, are released or emitted by atoms or subatomic particles. This emission occurs when an atom or particle transitions from a higher energy state to a lower energy state. The release of photons can occur in various ways, including through spontaneous emission, stimulated emission, or as a result of electronic transitions within atoms or molecules. Photon emission has a wide range of applications, from the generation of light in light bulbs and lasers to the emission of photons in biological processes such as bioluminescence. Understanding photon emission is crucial in many areas of science and technology, from quantum physics to telecommunications. In this article, we will explore the concept of photon emission in more detail.
Definition of Photon Emission
Photon emission refers to the process by which a photon, a fundamental particle of light, is released or emitted from an atom, molecule, or other physical system. This emission can occur due to various mechanisms, including spontaneous emission, stimulated emission, or fluorescence.
Spontaneous emission occurs when an excited atom or molecule transitions from a higher energy state to a lower energy state, spontaneously releasing a photon in the process. This emission is random and independent of external influences.
Stimulated emission, on the other hand, occurs when an incoming photon triggers an excited atom or molecule to release an additional photon that is identical in energy, phase, and direction to the incoming photon. This process is the basis for the amplification of light in lasers.
Fluorescence is a specific form of photon emission that occurs when a substance absorbs photons and subsequently re-emits them at a lower energy level. This emission usually occurs with a slight delay after the absorption and can be used for various applications, such as diagnostic imaging or fluorescent microscopy.
In all these processes, photon emission plays a crucial role in transferring energy and information through the exchange of photons, and it lies at the heart of many important phenomena in physics and technology.
Mechanisms of Photon Emission
Photon emission is the process by which a photon is emitted or released from an atom, molecule, or other particle. It occurs when an electron in an excited state transitions to a lower energy level, resulting in the release of energy in the form of a photon.
There are several mechanisms through which photon emission can occur:
1. Spontaneous emission: This is the most common mechanism of photon emission, where an electron transitions from a higher energy level to a lower energy level without any external influence. The transition occurs randomly, and the emitted photons have various energies, resulting in a broad spectrum of emitted light.
2. Stimulated emission: This process involves the interaction of a photon with an excited electron in an atom or molecule. When a photon with the same energy (frequency) as the transition energy of the electron interacts with it, it stimulates the electron to drop to a lower energy level, releasing two photons that are identical in terms of energy, phase, and direction. Stimulated emission is the basis of laser amplification and is responsible for the coherence and directionality of laser light.
3. Fluorescence: This mechanism occurs when an electron in an atom or molecule absorbs a photon with sufficient energy to excite it to a higher energy level. The excited electron rapidly loses energy and returns to its ground state by emitting a lower-energy photon. This emitted photon is usually of a longer wavelength (lower energy) than the absorbed photon.
4. Phosphorescence: Similar to fluorescence, phosphorescence involves the absorption of a photon by an electron, resulting in its excitation to a higher energy level. However, in phosphorescence, the return of the electron to its ground state is delayed, often spanning from milliseconds to hours. The emitted photon has lower energy and longer wavelength than the absorbed photon.
5. Bioluminescence: This is a process found in living organisms, where chemical reactions within cells or organisms produce excited states that result in photon emission. Examples include fireflies, jellyfish, and certain bacteria that emit light.
These mechanisms of photon emission play crucial roles in various fields, including optics, laser technology, spectroscopy, and the understanding of light-matter interactions.
Applications of Photon Emission
Photon emission refers to the process in which a photon is absorbed by an atom or molecule, causing its electrons to transition to a lower energy state and subsequently emit another photon. This phenomenon has various applications in different fields, including:
1. Laser technology: In lasers, the process of photon emission is utilized to produce coherent and concentrated beams of light. Stimulated photon emission, where photons stimulate the emission of identical photons, is essential for achieving the amplification and coherence required in laser systems.
2. Lighting technology: Fluorescent and phosphorescent lights utilize the principle of photon emission. When electricity passes through a gas or vapor, it excites the atoms or molecules, causing them to emit photons of specific wavelengths. These photons contribute to the visible light emitted by the bulb.
3. Solar cells: Photon emission plays a crucial role in solar cells by converting incident photons into electrical energy. The photons absorbed by the photovoltaic material excite the electrons, leading to a flow of electricity. This process enables the harnessing of solar energy for various applications, including power generation.
4. Imaging and photography: The detection of emitted photons is fundamental in various imaging techniques and photography. Sensors, such as charge-coupled devices (CCDs) or complementary metal-oxide-semiconductor (CMOS) sensors, can detect and capture photons to create images in digital cameras, telescopes, and other imaging devices.
5. Medical diagnostics: Photon emission is used in medical imaging, such as positron emission tomography (PET). In PET scans, radioisotopes emit positrons, which annihilate with nearby electrons, producing two gamma photons. These emitted photons are detected by the scanners, enabling the mapping of tissues and the diagnosis of medical conditions.
6. Quantum communication: In quantum communication systems, entangled photons are employed to transmit secure information. Photon emission and detection play a crucial role in establishing and maintaining the entanglement between photons, which enables secure quantum key distribution and quantum teleportation.
7. Atomic and molecular physics: By studying the spectral lines emitted during photon emission, scientists can gain insight into the energy levels and transitions occurring within atoms and molecules. This knowledge helps in understanding atomic and molecular structures and processes, aiding scientific research in various fields.
Overall, photon emission has a wide range of applications in technology, energy conversion, imaging, medical diagnostics, and scientific research. Its understanding and control enable numerous advancements across different disciplines.
Conclusion
In conclusion, photon emission refers to the process in which photons are generated and emitted by atoms, molecules, or other subatomic particles. This emission can occur through various mechanisms, such as the transition of electrons between energy states or the decay of excited states. Photon emission plays a crucial role in a wide range of phenomena, including light and electromagnetic wave generation, fluorescence, bioluminescence, and many other optical processes. Understanding and controlling photon emission is essential in fields such as physics, chemistry, and engineering, and has important applications in technologies such as lasers, LED lights, and optical communications.
Topics related to Photon emission
Energy Levels & Emission Spectra – A-level Physics – YouTube
Energy Levels & Emission Spectra – A-level Physics – YouTube
Emission and Absorption Line Spectra – A Level Physics – YouTube
Emission and Absorption Line Spectra – A Level Physics – YouTube
Stimulated Emission – YouTube
Stimulated Emission – YouTube
Emission and Absorption Spectra – YouTube
Emission and Absorption Spectra – YouTube
Electron Energy Levels and Photons – IB Physics – YouTube
Electron Energy Levels and Photons – IB Physics – YouTube
Absorption and Emission Spectra – IB Physics – YouTube
Absorption and Emission Spectra – IB Physics – YouTube
Emission and Absorption Line Spectra – A Level Physics – YouTube
Emission and Absorption Line Spectra – A Level Physics – YouTube
Monster from Outer Space: BLACK HOLE | Big Documentary. – YouTube
Monster from Outer Space: BLACK HOLE | Big Documentary. – YouTube
How Does Light Actually Work? – YouTube
How Does Light Actually Work? – YouTube
From Birth to Death: Black holes – The Cosmic Phenomena Beyond Our Galaxy. – YouTube
From Birth to Death: Black holes – The Cosmic Phenomena Beyond Our Galaxy. – YouTube

Konstantin Sergeevich Novoselov is a Russian-British physicist born on August 23, 1974. Novoselov is best known for his groundbreaking work in the field of condensed matter physics and, in particular, for his co-discovery of graphene. Novoselov awarded the Nobel Prize in Physics. Konstantin Novoselov has continued his research in physics and materials science, contributing to the exploration of graphene’s properties and potential applications.