Introduction
Introduction:
Diazomethane acid, also known as diazomethane carboxylic acid or diazomethylsulfuric acid, is a chemical compound that is encountered primarily in organic synthesis. It is a precursor to the highly reactive and potentially explosive compound diazomethane (CH2N2), which is widely used in organic chemistry as a methylating agent.
Diazomethane acid is typically obtained as a solid or as a colorless liquid, depending on the conditions of preparation. It is highlypity acidic in nature, owing to the presence of carboxylic acid or sulfuric acid groups. As a result, it can behave as a strong acid in certain reactions.
This compound is chemically unstable and sensitive to heat, shock, and friction. It decomposes easily, often explosively, to release hazardous gases and vapors, particularly nitrogen gas. Therefore, it requires careful handling and should only be used in specially equipped laboratories by trained personnel.
Despite its potential hazards, diazomethane acid has found applications in various areas of organic synthesis. It can be used to prepare diazomethane, which is useful in reactions such as the Wolff rearrangement and the Curtius rearrangement. Diazomethane itself is important in the synthesis of various compounds, including pharmaceuticals, agrochemicals, and dyes.
In conclusion, diazomethane acid is a chemical compound that serves as a precursor to the highly reactive compound diazomethane. Although it is unstable and potentially dangerous, it has valuable applications in organic synthesis. Careful handling and strict safety precautions are necessary when working with this compound.
Properties of Diazomethane Acid
Diazomethane is not an acid. It is a highly reactive and potentially explosive compound that is often used in organic chemistry as a carbene source. Diazomethane is a yellow gas that is usually stored and used as a solution in ether or other organic solvents.
Some important properties of Diazomethane are:
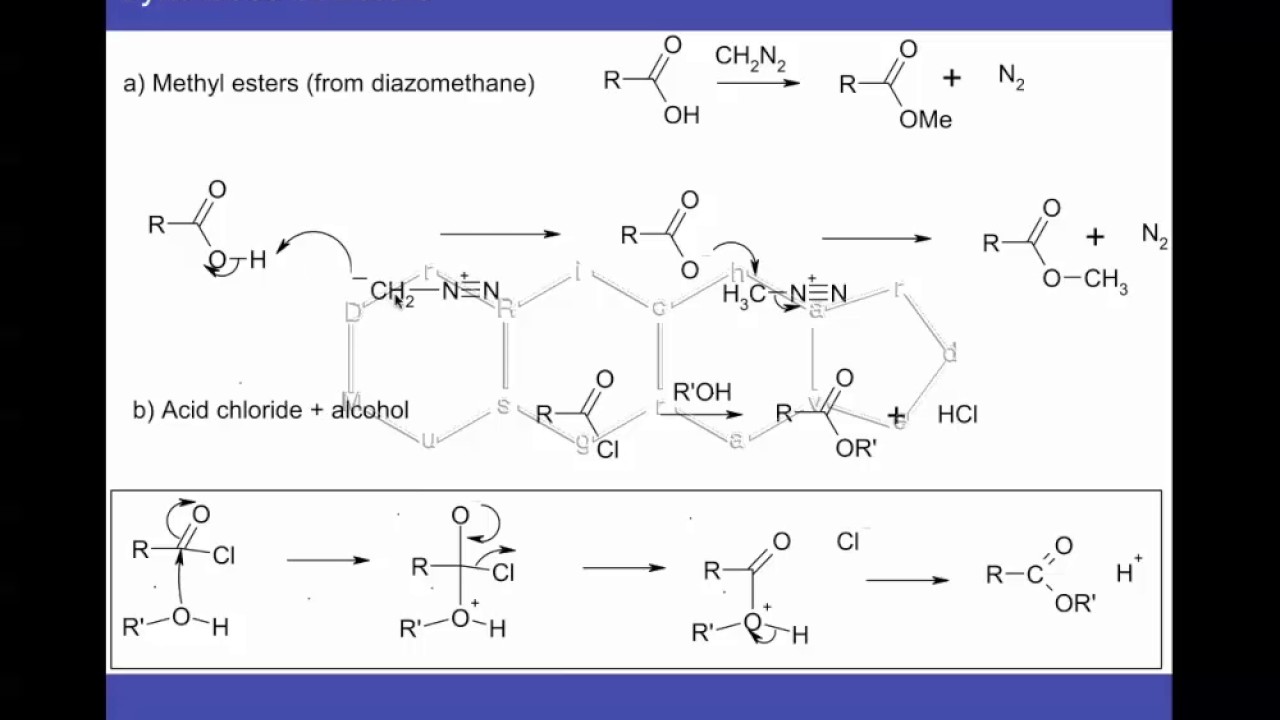
1. Reactivity: Diazomethane is highly reactive and can undergo a variety of reactions with different organic compounds. It is most commonly used to convert carboxylic acids into their methyl esters.
2. Explosive nature: Diazomethane is highly explosive and must be handled with extreme precautions. It can easily decompose, leading to the formation of nitrogen gas and highly reactive carbene species, which can initiate explosive reactions.
3. Stability: Diazomethane is relatively unstable and can decompose spontaneously over time, especially under light or elevated temperature conditions. The decomposition can result in the formation of hazardous byproducts. Therefore, it is typically prepared and used immediately.
4. Toxicity: Diazomethane is toxic and can be harmful if inhaled, ingested, or comes in contact with the skin or eyes. It is classified as a potential carcinogen and should be handled in a fume hood with appropriate protective measures.
Overall, due to its high reactivity, explosiveness, instability, and toxicity, Diazomethane requires careful handling and should only be used by experienced chemists in a controlled laboratory setting.
Uses of Diazomethane Acid in Chemistry
Diazomethane, CH2N2, is a compound primarily used in organic chemistry as a reagent for various reactions. However, there is no such thing as “diazomethane acid” in chemistry. Diazomethane is a neutral compound and not an acid. Therefore, let’s focus on the uses of diazomethane in chemistry:
1. Methylation: Diazomethane is commonly used for the methylation of various organic compounds. It can transfer a methyl group (-CH3) to nucleophiles, such as alcohols, amines, and carboxylic acids, leading to the formation of methylation products.
2. Cyclopropanation: Diazomethane is a valuable reagent for the synthesis of cyclopropanes, which are three-membered rings containing two carbon atoms and one heteroatom. It can react with alkenes or carbonyl compounds to form cyclopropane derivatives.
3. Wolff Rearrangement: Diazomethane can undergo the Wolff rearrangement, which involves intramolecular migration of a carbene group. This reaction is useful for the synthesis of ketenes, which are important intermediates in various organic transformations.
4. Wittig Reaction: Diazomethane can be used as a precursor to generate a carbene species, which can then undergo the Wittig reaction with aldehydes or ketones. This reaction allows for the synthesis of alkenes by the formation of carbon-carbon double bonds.
5. Diazotization: Diazomethane can be used to diazotize primary aromatic amines to form diazonium salts. These diazonium salts are versatile intermediates that allow for the synthesis of various aromatic compounds, such as azo dyes and aryl halides.
It is important to note that diazomethane is highly reactive and potentially explosive. Therefore, it should be handled with extreme caution and only by trained personnel in a well-equipped laboratory.
Safety Considerations and Handling of Diazomethane Acid
Diazomethane is an extremely hazardous compound that should be handled with extreme caution. It is a yellow gas that is highly flammable and explosive. It is also toxic and can cause severe burns and damage to the respiratory system if inhaled.
When working with diazomethane, it is important to follow strict safety guidelines:
1. Personal Protective Equipment (PPE): Wear appropriate PPE, including gloves, goggles, and a lab coat, to protect yourself from potential exposure.
2. Fume Hood: Perform all operations involving diazomethane in a well-ventilated fume hood to minimize inhalation exposure.
3. Storage: Diazomethane should be stored in a cool, dark place, preferably in a sealed container to prevent any release of the gas. It should be stored away from sources of ignition, heat, and incompatible materials.
4. Handling: Diazomethane should only be handled by experienced personnel who are familiar with its properties and associated risks. Use specialized equipment, such as syringes or cannulae, when transferring or dispensing diazomethane.
5. Stability: Diazomethane is highly unstable and can decompose explosively upon exposure to heat, light, or friction. Do not subject it to any rough handling or sudden changes in temperature.
6. Chemical Incompatibility: Diazomethane is incompatible with a wide range of substances, including strong oxidizers, strong acids, bases, and heavy metals. Avoid contact with these materials to prevent potential reactions or explosions.
7. Waste Disposal: Diazomethane waste should be carefully collected and disposed of according to local regulations. It should never be flushed down the sink or disposed of in regular waste streams.
It is strongly recommended to conduct work with diazomethane under the guidance of an experienced chemist and to refer to the specific safety data sheet (SDS) provided by the manufacturer for detailed handling instructions and precautions.
Conclusion
In conclusion, diazomethane is an acidic compound.
Topics related to Diazomethane acid
Diazomethane formation of methyl esters – YouTube
Diazomethane formation of methyl esters – YouTube
Diazomethane Synthesis and Applications (Arndt-Eistert Homologation) – YouTube
Diazomethane Synthesis and Applications (Arndt-Eistert Homologation) – YouTube
Diazomethane Reactions to form Methyl Esters – YouTube
Diazomethane Reactions to form Methyl Esters – YouTube
Diazomethane – YouTube
Diazomethane – YouTube
OQV NO – 146 Reaction of acetic acid with diazomethane. – YouTube
OQV NO – 146 Reaction of acetic acid with diazomethane. – YouTube
Reactions of Carboxylic Acids: Esterification Using Diazomethane – YouTube
Reactions of Carboxylic Acids: Esterification Using Diazomethane – YouTube
Cyclopropanation of Alkenes Carbene via Haloform and Simmons Smith Reactions – YouTube
Cyclopropanation of Alkenes Carbene via Haloform and Simmons Smith Reactions – YouTube
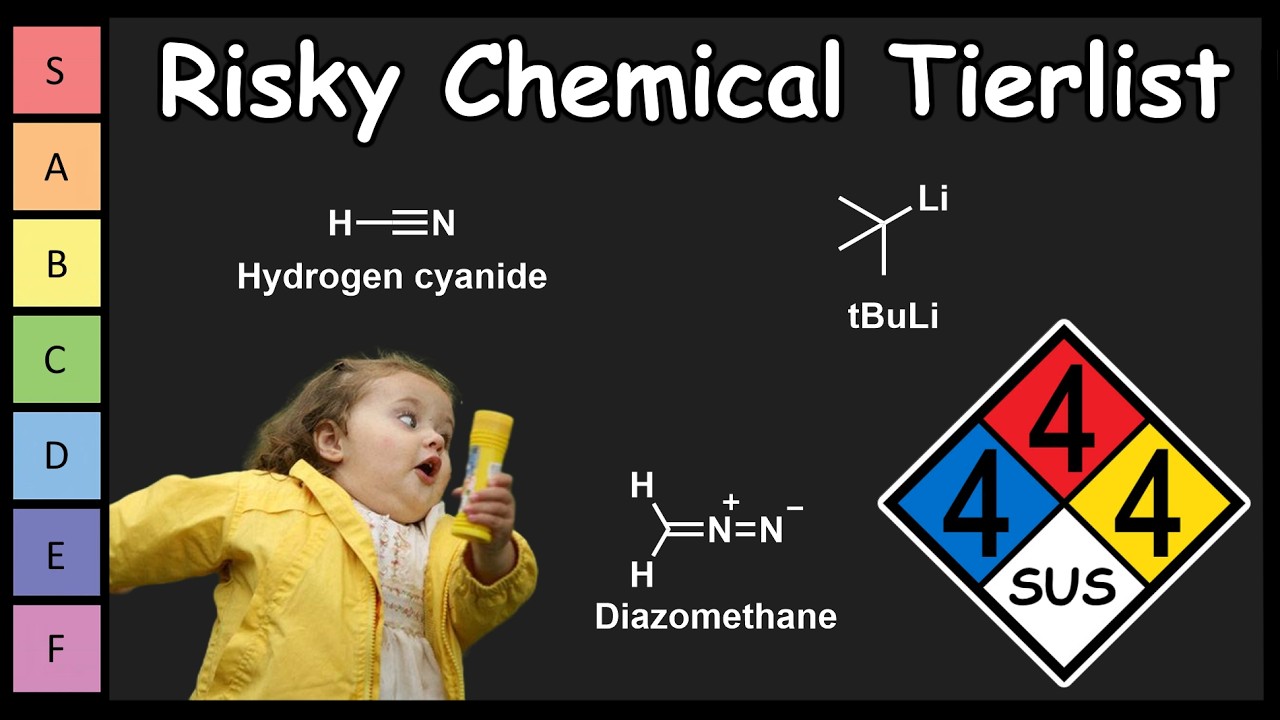
Which Chemical is the Most Risky? – YouTube
Which Chemical is the Most Risky? – YouTube
The strengths and weaknesses of acids and bases – George Zaidan and Charles Morton – YouTube
The strengths and weaknesses of acids and bases – George Zaidan and Charles Morton – YouTube
An Overview of Aldehydes and Ketones: Crash Course Organic Chemistry #27 – YouTube
An Overview of Aldehydes and Ketones: Crash Course Organic Chemistry #27 – YouTube

Abigail Gutmann Doyle is a renowned Organic chemistry professor in Los Angeles. Her research focuses on the development of new chemical transformations in organic chemistry. She has won awards such as: Bayer Early Excellence in Science Award, Phi Lambda Upsilon National Fresenius Award, Presidential Early Career Award for Scientists and Engineers, BMS Unrestricted Grant in Synthetic Organic Chemistry.