Introduction to Nuclear Magnetic Resonance Spectroscopy
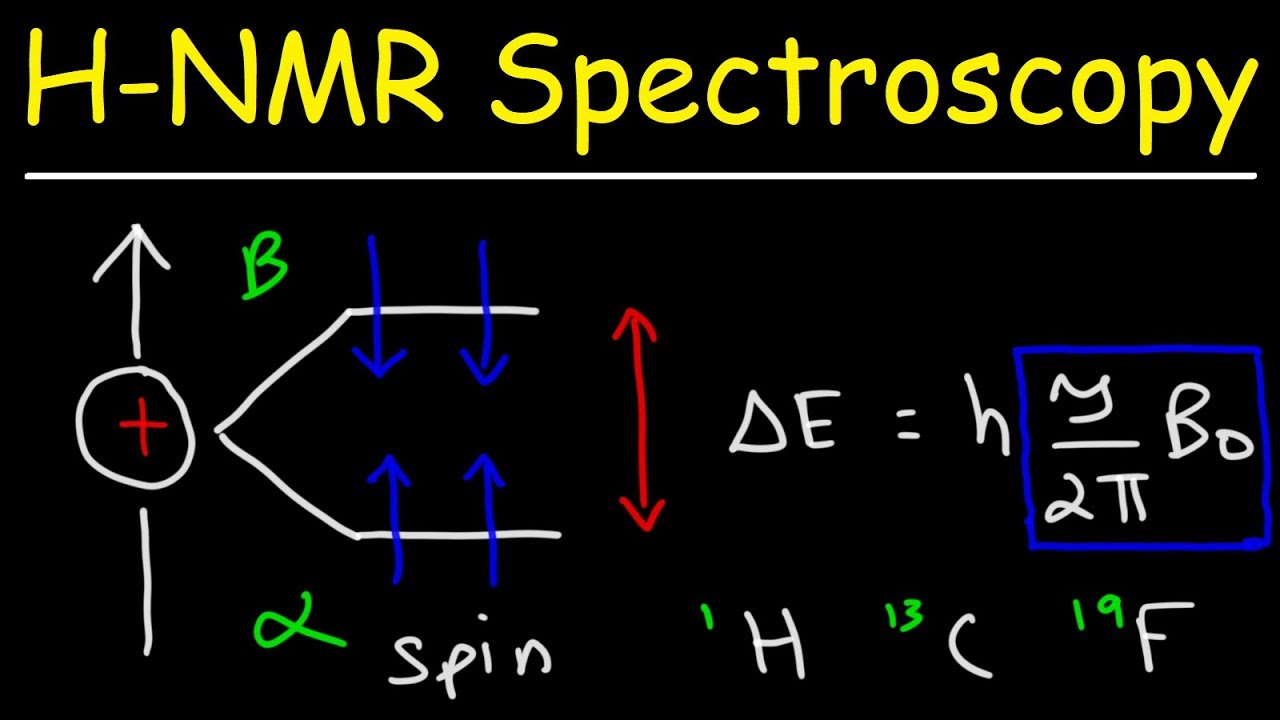
Nuclear Magnetic Resonance (NMR) spectroscopy is a powerful analytical technique used to study the physical and chemical properties of molecules. It is based on the interaction between the magnetic field and the spin properties of atomic nuclei.
In NMR spectroscopy, the sample under study is placed in a strong magnetic field. This causes the atomic nuclei within the sample to align either with or against the magnetic field. When a radiofrequency pulse is applied to the sample, it can excite the nuclei from their lower energy state to a higher energy state, causing them to resonate.
As the excited nuclei return to their lower energy state, they emit energy in the form of electromagnetic radiation, which can be detected and analyzed to provide valuable information about the molecular structure and dynamics of the sample.
The NMR spectrum obtained from a sample contains a series of peaks that correspond to different resonating nuclei within the molecule. The positions of these peaks are influenced by factors such as the chemical environment, neighboring atoms, and molecular symmetry.
NMR spectroscopy is widely used in various fields of science, including chemistry, biochemistry, and medicine. It can provide information about molecular structures, determine the concentrations of different components in a mixture, study reaction kinetics, and even non-invasively image tissues in medical imaging techniques such as Magnetic Resonance Imaging (MRI).
Overall, NMR spectroscopy is a versatile and non-destructive analytical technique that offers valuable insights into the structure and behavior of molecules, making it an essential tool for researchers in a wide range of scientific disciplines.
Principles of Nuclear Magnetic Resonance Spectroscopy
Nuclear Magnetic Resonance (NMR) spectroscopy is a powerful analytical technique used to study the structure, composition, and dynamics of molecules. It is based on the principle of nuclear magnetic resonance, which involves the interaction of atomic nuclei (specifically those with an odd number of protons or neutrons) with an applied magnetic field.
The principles of NMR spectroscopy are as follows:
1. Resonance: NMR relies on the resonance phenomenon where the atomic nuclei absorb and emit energy at specific frequencies when subjected to a magnetic field. The resonance frequency is determined by the type and chemical environment of the nuclei.
2. Chemical Shift: The chemical shift is the measurement of the resonance frequency of a nucleus relative to a reference compound. It is affected by the electronic environment around the nucleus, providing information about the chemical structure.
3. Spin-Spin Coupling: This principle describes the interaction between nuclei that have nonzero spin. It causes splitting of NMR signals into multiple peaks, known as multiplets. Spin-spin coupling provides information about the connectivity and arrangement of atoms in a molecule.
4. Relaxation: After absorbing energy, the excited nuclear spins return to their equilibrium state by releasing the excess energy. This process is referred to as relaxation and has two components: T1 relaxation (longitudinal relaxation) and T2 relaxation (transverse relaxation). These relaxation times provide information about molecular motion and molecular interactions.
5. Integration: The area under NMR peaks in a spectrum corresponds to the number of nuclei responsible for each peak. Integration analysis allows for quantitative determination of the relative amounts of different nuclei present in a sample.
6. Spectral Analysis: Spectral analysis involves the interpretation of NMR spectra to deduce structural information about molecules. By analyzing peak positions, intensities, splitting patterns, and other factors, valuable information about the chemical environment, connectivity, and stereochemistry of atoms can be obtained.
Overall, NMR spectroscopy works by measuring the energy absorption and emission of atomic nuclei in a magnetic field, providing valuable information about molecular structures and dynamics. This technique is widely used in various fields, including organic and inorganic chemistry, biochemistry, pharmaceutical research, and materials science.
Applications of Nuclear Magnetic Resonance Spectroscopy
Nuclear magnetic resonance (NMR) spectroscopy is a powerful analytical technique used to study the structure, composition, and dynamics of molecules. It is based on the interaction between the magnetic field and the nuclei of atoms within a sample.
Applications of NMR spectroscopy include:
1. Structural elucidation: NMR spectroscopy is commonly used to determine the structure of organic compounds. By analyzing the chemical shifts, splitting patterns, and coupling constants observed in NMR spectra, scientists can deduce the connectivity and arrangement of atoms within a molecule.
2. Compound identification: NMR spectroscopy can be used to identify unknown compounds. Comparing the NMR spectrum of an unknown compound to a library of reference spectra allows for identification based on characteristic peaks and chemical shifts.
3. Quantitative analysis: NMR spectroscopy can be used to quantitatively measure the concentration of certain compounds within a mixture. By comparing the integration of specific NMR peaks to a known standard, the amount of a particular compound can be determined.
4. Reaction monitoring: NMR spectroscopy can be used to monitor chemical reactions in real-time. By acquiring NMR spectra at different time intervals, the progress of a reaction and the formation of intermediates or by-products can be tracked.
5. Protein structure determination: NMR spectroscopy can be used to study the structure and dynamics of proteins. By labeling certain amino acids with isotopes and observing the resulting NMR signal, information about the folding, conformation, and interactions of proteins can be obtained.
6. Drug discovery and development: NMR spectroscopy plays a crucial role in drug discovery and development. It can be used to study the interaction between drugs and their targets (e.g., proteins), helping scientists understand the binding affinity, binding site, and mode of action of drugs.
7. Environmental analysis: NMR spectroscopy can be used to analyze environmental samples such as water and soil. It can provide information about the presence and concentration of pollutants, organic compounds, and contaminants.
8. Material science: NMR spectroscopy is used in the characterization and analysis of materials, including polymers, catalysts, and nanoparticles. It can provide information about the molecular composition, structure, and properties of materials.
Overall, NMR spectroscopy has diverse applications in various fields, including chemistry, biochemistry, pharmaceuticals, materials science, and environmental science. Its non-destructive nature, high resolution, and ability to provide quantitative and structural information make it a valuable analytical technique.
Techniques and Instrumentation in Nuclear Magnetic Resonance Spectroscopy
Nuclear Magnetic Resonance (NMR) spectroscopy is a powerful analytical technique used to investigate the chemical and physical properties of molecules. It provides detailed information about the molecular structure, dynamics, and interactions. NMR spectroscopy operates based on the interaction between atomic nuclei possessing a magnetic moment and an external magnetic field. The technique has found applications in various fields, including chemistry, biochemistry, pharmaceuticals, and materials science.
Techniques:
1. Continuous Wave (CW) Technique: In CW-NMR, a continuous radiofrequency (RF) signal is applied to the sample, and the resulting NMR signal is detected and analyzed. This technique is relatively simple and commonly used for routine analysis.
2. Pulsed NMR Technique: Pulsed NMR involves applying a series of RF pulses to the sample and measuring the resulting NMR signals. Various pulse sequences, such as spin-echo and inversion-recovery sequences, can be used to obtain specific information about the molecule under study, such as chemical shift, spin-spin coupling, and relaxation times.
3. Fourier Transform (FT) Technique: In FT-NMR, a series of free induction decays (FIDs) obtained after applying a short RF pulse is transformed into a frequency domain using a mathematical technique called Fourier transform. FT-NMR provides high spectral resolution and is widely used due to its ability to rapidly acquire high-quality spectra.
Instrumentation:
1. Magnet: NMR spectroscopy requires a strong and homogeneous magnetic field for accurate measurements. Superconducting magnets are commonly used, which can generate magnetic fields ranging from a few Tesla to over 20 Tesla.
2. Probe: The NMR probe is the part of the instrument that interacts with the sample. It typically consists of a coil for transmitting the RF pulse and another coil for receiving the resulting NMR signal. Probes can be designed for different sample types, such as liquids, solids, or gases.
3. RF System: The RF system generates the necessary RF pulses and controls their timing and amplitude. It consists of a transmitter, receiver, and various RF amplifiers and filters. The RF system is crucial for controlling the excitation, acquisition, and manipulation of NMR signals.
4. Data Acquisition System: The NMR signals are digitized by the data acquisition system, which records the time-domain signal (FID) and performs data processing, including Fourier transform and signal analysis.
5. Spectrometer: The spectrometer is the central component of NMR instrumentation that integrates all the necessary components, such as the magnet, probe, RF system, and data acquisition system. It controls the overall experiment, including pulse sequences, data acquisition, and analysis.
In conclusion, NMR spectroscopy involves various techniques and instrumentation for the analysis of molecular structure and dynamics. The continuous wave and pulsed techniques, combined with Fourier transform analysis, offer a comprehensive understanding of chemical shifts, spin-spin couplings, and relaxation behavior. The choice of magnet, probe design, RF system, and spectrometer are critical for acquiring accurate and high-quality NMR spectra.
Limitations and Challenges in Nuclear Magnetic Resonance Spectroscopy
Limitations and Challenges in Nuclear Magnetic Resonance (NMR) Spectroscopy
1. Sensitivity: NMR spectroscopy requires a large number of nuclei to generate a detectable signal. This limits the sensitivity of NMR, especially when studying compounds with low abundance or in small quantities.
2. Signal Overlap: In complex mixtures, the signals from different nuclei may overlap, making it difficult to assign specific frequencies to individual nuclei. This can lead to challenges in interpreting the resulting NMR spectra.
3. Signal-to-Noise Ratio: Both coherent and incoherent noise can affect the quality of NMR spectra, resulting in a decrease in the signal-to-noise ratio. This can make it challenging to extract meaningful information from the spectra and may require longer acquisition times.
4. Spectral Resolution: The resolution of NMR peaks can be limited by various factors, such as magnetic field inhomogeneity and broadening effects. This can lead to overlapping peaks and difficulties in accurately determining chemical shifts and coupling constants.
5. Sample Preparation: NMR spectroscopy often requires samples to be dissolved in a suitable solvent. However, some compounds may not readily dissolve or may interact with the solvent, thereby affecting their spectral characteristics. Additionally, the choice of solvent can impact the solubility and stability of the sample.
6. Instrumentation: NMR spectroscopy requires high-quality and well-calibrated instrumentation for accurate and reliable results. Issues with instrument stability, homogeneity, and calibration can affect the quality of NMR spectra and may require expert intervention to resolve.
7. Computational Challenges: Interpretation and analysis of NMR spectra often involve complex data processing and modeling. This requires advanced computational techniques and algorithms, which can be challenging and time-consuming.
8. Sensitivity to Paramagnetic Species: NMR spectroscopy is not well-suited for studying paramagnetic compounds or species containing unpaired electrons. The presence of paramagnetic centers can disrupt the local magnetic field, leading to broadened and distorted NMR signals.
9. Time and Cost: NMR spectroscopy can be time-consuming, especially for complex samples or when high-resolution spectra are required. Additionally, the cost of NMR instrumentation and maintenance can be significant, limiting its accessibility in some research settings.
In summary, although NMR spectroscopy is a powerful analytical technique, it faces several limitations and challenges that need to be considered when designing experiments and interpreting NMR spectra. Overcoming these limitations often requires careful sample preparation, advanced data analysis techniques, and high-quality instrumentation.
Topics related to Nuclear magnetic resonance spectroscopy
Basic Introduction to NMR Spectroscopy – YouTube
Basic Introduction to NMR Spectroscopy – YouTube
What's Nuclear Magnetic Resonance (NMR)? How Does It Work? What's It Used For? A Brief Introduction. – YouTube
What's Nuclear Magnetic Resonance (NMR)? How Does It Work? What's It Used For? A Brief Introduction. – YouTube
NMR spectroscopy visualized – YouTube
NMR spectroscopy visualized – YouTube
What is Nuclear Magnetic Resonance (NMR)? // HSC Chemistry – YouTube
What is Nuclear Magnetic Resonance (NMR)? // HSC Chemistry – YouTube
Nuclear Magnetic Resonance: Principles and Applications of NMR – YouTube
Nuclear Magnetic Resonance: Principles and Applications of NMR – YouTube
NMR Spectroscopy| Chemical shift | Shielding & Deshielding | Equivalent Non-Equivalent Protons | TMS – YouTube
NMR Spectroscopy| Chemical shift | Shielding & Deshielding | Equivalent Non-Equivalent Protons | TMS – YouTube
THE ORIGIN OF LIGHT – YouTube
THE ORIGIN OF LIGHT – YouTube
Introduction to NMR spectroscopy – YouTube
Introduction to NMR spectroscopy – YouTube
How MRI Works – Part 1 – NMR Basics – YouTube
How MRI Works – Part 1 – NMR Basics – YouTube
The first SCIENCE IMAGES from the Euclid Space Telescope: all the details! | Night Sky News Nov 2023 – YouTube
The first SCIENCE IMAGES from the Euclid Space Telescope: all the details! | Night Sky News Nov 2023 – YouTube

Konstantin Sergeevich Novoselov is a Russian-British physicist born on August 23, 1974. Novoselov is best known for his groundbreaking work in the field of condensed matter physics and, in particular, for his co-discovery of graphene. Novoselov awarded the Nobel Prize in Physics. Konstantin Novoselov has continued his research in physics and materials science, contributing to the exploration of graphene’s properties and potential applications.